Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas : A Prospective Multicenter Study of Korean Patients
Article information
Abstract
Objective
This study was performed to determine the safety and outcome of concurrent chemoradiotherapy (CCRT) and adjuvant chemotherapy with temozolomide for Korean patients with a newly diagnosed glioblastoma.
Methods
Patients were recruited from four institutions between 2004 and 2007. The patients received fractionated focal irradiation in daily fractions of 2 Gy given 5 days per week for 6 weeks and daily temozolomide, followed by 6 cycles of adjuvant temozolomide. The primary endpoint was overall survival (OS) and the secondary endpoints were progression-free survival (PFS), response, and safety.
Results
A total of 103 patients were enrolled in this study. Ninety-six patients (93%) completed the CCRT and 54 patients (52%) received 6 cycles of adjuvant temozolomide. The response rate was 73% (53/73) and the tumor control rate was 92% (67/73). Of the 96 patients who completed the CCRT, the median OS was 18.0 months and the 1- and 2-year OS rates were 74 and 38%, respectively. The median PFS was 10.0 months and the 1- and 2-year PFS rates were 33 and 16%, respectively. The only significant prognostic factor of survival was the extent of surgical resection (p<0.05). CCRT resulted in grade 3 or 4 hematologic toxic effects in 8% of patients. No opportunistic infections were noted.
Conclusion
This study is the first prospective multi-institutional report of CCRT and adjuvant chemotherapy with temozolomide for patients with a newly diagnosed glioblastoma in Korea. The current protocol may prolong the survival of Korean patients with a glioblastoma and may be tolerable in terms of toxicity.
INTRODUCTION
Glioblastoma, a frequent primary intracranial malignant tumor, presents with a dismal prognosis despite multimodal treatment modalities, such as surgery, radiotherapy and chemotherapy4). Most patients who are diagnosed with primary glioblastoma die within two years even in the most favorable situations. Recently, a number of studies and trials have been performed to develop new drugs and interventions against this terrible disease.
In 2005, Stupp et al.17,18) reported the results of the first prospective multi-institutional randomized controlled trial of concurrent chemoradiotherapy (CCRT) followed by adjuvant chemotherapy with temozolomide against primary glioblastomas and demonstrated that this treatment protocol prolonged the overall survival (OS) and progression-free survival (PFS) of patients when compared with the previous standard treatment. Since that report, CCRT and adjuvant chemotherapy with temozolomide have been recognized as the primary treatment for newly diagnosed glioblastoma, and temozolomide as an essential chemotherapeutic drug for primary glioblastoma treatment13).
Therefore, we designed a prospective multicenter trial to confirm that if Stupp's treatment protocol was also effective and safe in Korean patients with newly diagnosed glioblastomas. The aims of this study were to settle on a standard treatment protocol for Korean patients with primary glioblastomas and to gather control group data for future randomized comparative trials.
MATERIALS AND METHODS
Patients
Patients over the age of 17 with a newly diagnosed and histopathologically confirmed glioblastoma were enrolled from 4 institutions. Eligible patients had a Karnofsky Performance Scale (KPS) score ≥60 and adequate hematological, renal, and hepatic functions, which were defined as an absolute neutrophil count ≥1500/µL, a platelet count ≥100000/µL, hemoglobin ≥10 g/dL, serum creatinine and total serum bilirubin levels less than 1.5 times the upper limit of the normal range, aspartate aminotransferase and alanine aminotransferase levels less than 2.5 times the upper limit of the normal range, and alkaline phosphatase levels less than 2 times the upper limit of the normal range. All patients were required to sign a form giving their fully informed consent to take part in the study, which was approved by the respective institutional review boards of the four institutions.
Treatment protocols
The patients enrolled in this study were treated with CCRT and adjuvant chemotherapy using temozolomide with the Stupp's regimen17,18). Within 6 weeks after the histological diagnosis of glioblastoma, the patients were assigned to receive CCRT with temozolomide. According to this protocol, the radiotherapy component consisted of fractionated focal irradiation at a dose of 2 Gy per fraction given once a day, 5 days a week, for a period of 6 weeks, for a total dose of 60 Gy. Temozolomide was given at a dose of 75 mg/m2/day, 7 days per week, from the first to the last day of radiotherapy and for no longer than 49 days. Temozolomide was administered daily 1 hour before radiotherapy or in the morning on days without radiotherapy.
After a 4-week break, the patients received up to six cycles of adjuvant temozolomide, according to the standard 5-day schedule, every 28 days. The temozolomide dose was 150 mg/m2 for the first cycle, and it was increased to 200 mg/m2 at the beginning of the second cycle if no hematological toxicity occurred. Prophylaxis for Pneumocystis jiroveci pneumonia was recommended during CCRT or if the lymphocyte counts decreased below 500/mm3.
Follow-up and evaluations
The baseline evaluations included a detailed patient history, a physical and neurological examination, KPS score determination, hematological and serological evaluation, and brain magnetic resonance (MR) images with gadolinium enhancement. MR scans were performed before the first adjuvant treatment cycle, every 2 or 3 months during the first year and every 3 months during the second year. The extent of resection was evaluated using immediate postoperative MR images within 72 hours after surgery. The gross total removal (GTR) was defined as >99% removal of the initial tumor, the subtotal removal (STR) as 50-99%, and the partial removal (PR) as <50%. Tumor progression was defined as a 25% increase in the maximal tumor area, the appearance of new lesions, and an increased corticosteroid need as previously reported12,19). When tumor progression occurred, the patients were managed at the discretion of the investigator, and the second-line treatment protocol was recorded. The patient's response to treatment was categorized into four groups by the modified WHO criteria as follows : complete remission, partial remission, stable disease, and progressive disease12,19). The toxic effects of treatment were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0), with a score of 1 indicating mild adverse effects, 2 indicating moderate adverse effects, 3 indicating severe adverse effects, 4 indicating life-threatening adverse effects, and 5 denoting death that was related to the adverse effect. The methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) promoter was evaluated using the methylation specific polymerase chain reaction.
Statistical analysis
The primary endpoint was overall survival (OS), and the secondary endpoints were progression-free survival (PFS) and safety. PFS was defined as the time from diagnosis to documented disease progression or death from any cause, whichever occurred first. OS was measured from the date of diagnosis to the date of death or the last follow-up examination. PFS and OS were estimated using the Kaplan-Meier method. Differences with regard to survival and disease progression were tested for significance using the two-sided log rank test. The Cox proportional hazards model was used to adjust for stratification factors and other confounding variables. All these analyses were performed using the SPSS statistical software package (release 17.0.1, 2008; SPSS, Chicago, IL, USA). The toxicities of temozolomide were reported separately for the radiotherapy period and the adjuvant chemotherapy period.
RESULTS
Patient characteristics and delivery of treatments
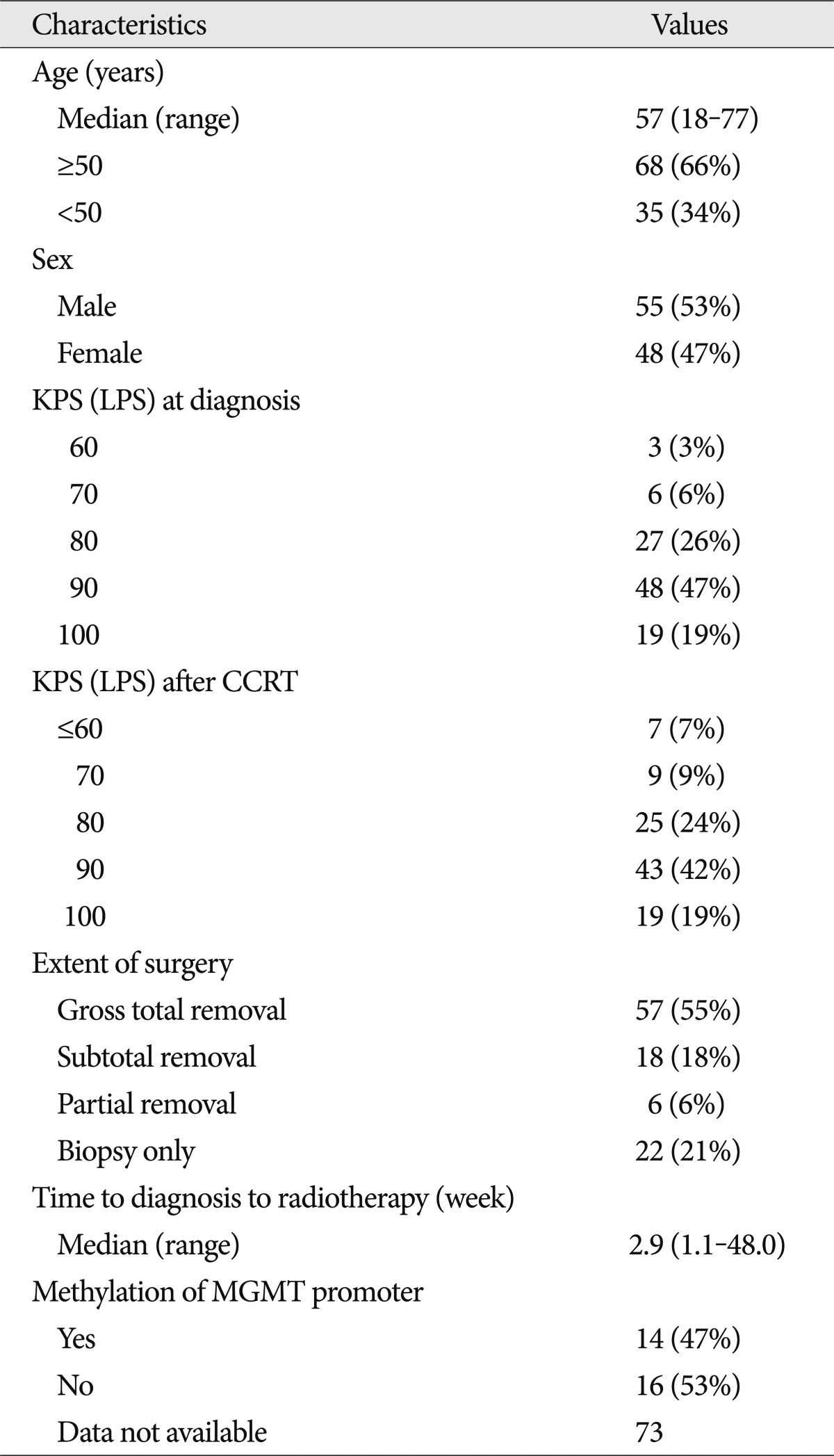
Between 2004 and 2007, 103 patients from four institutions were enrolled in this study. Their clinical characteristics at baseline are summarized in Table 1. The median age of the patients was 57 years (range, 18-77 years). Sixty-seven patients (65%) and 62 patients (60%) showed a KPS score of over 90 at diagnosis and after CCRT, respectively. Fifty-seven patients (55%) underwent GTR, 18 patients (18%) had STR, 6 patients (6%) experienced PR, and 22 patients (21%) had a biopsy only. Evaluation of the methylation of the MGMT promoter in 30 tumors revealed that 14 patients (47%) had MGMT promoter methylation.
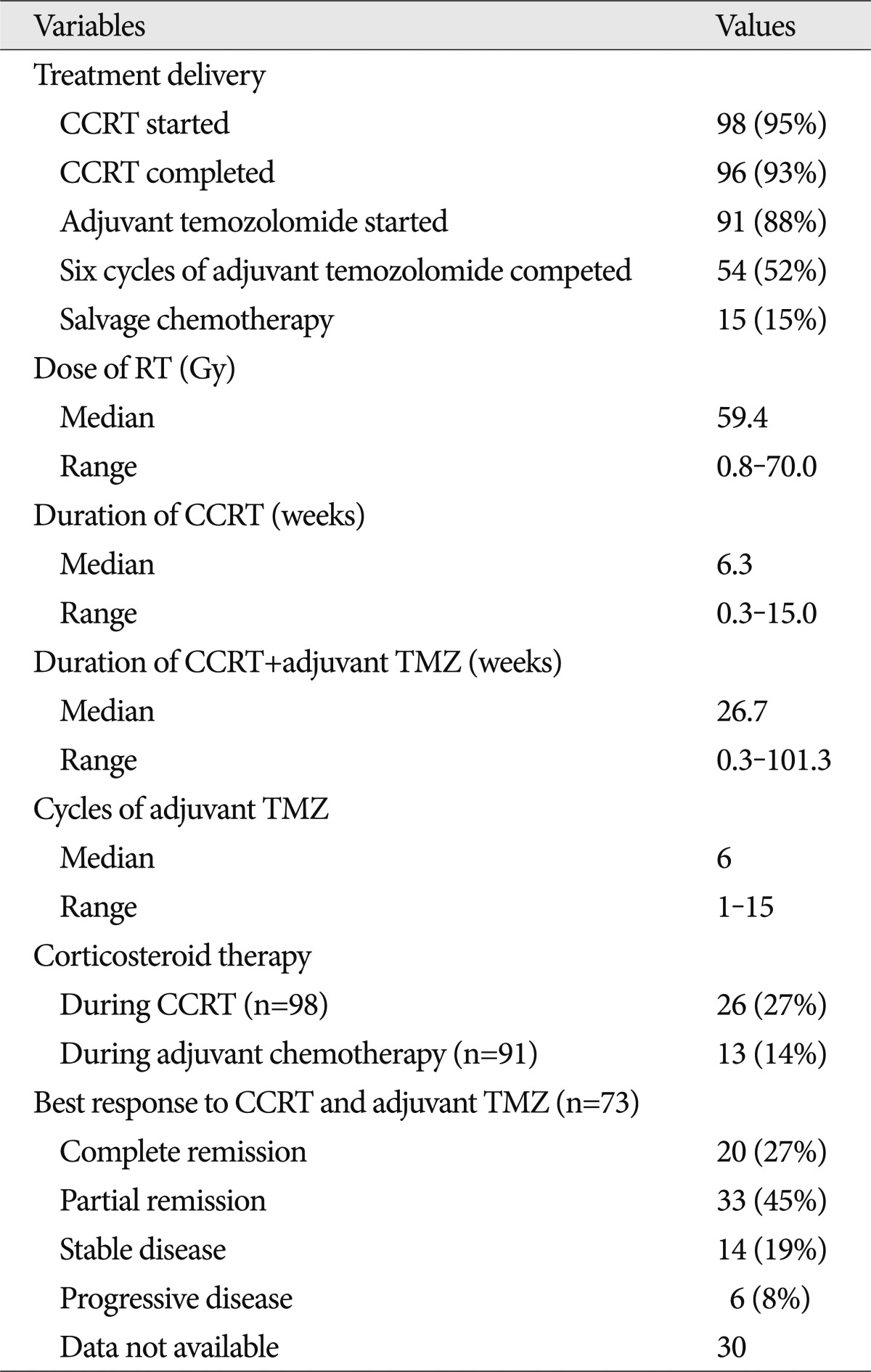
In this trial, 98 patients (95%) were given CCRT, and the remaining 5 patients were given adjuvant temozolomide as an initial therapy. Among the 98 patients, 2 patients were excluded because of loss at follow-up. Finally, 54 patients (52%) completed 6 cycles of adjuvant temozolomide, and 15 patients (15%) received salvage chemotherapy including nimustine (ACNU), lomustine (CCNU), cisplatin, avastin or irinotecan. Table 2 summarizes the details of the treatments administered to these patients. The median therapeutic radiation dose was 59.4 Gy (range, 0.8-70.0 Gy), and the median duration of CCRT was 6.3 weeks. The patients underwent a median of 6 cycles (range, 1-15) of adjuvant temozolomide, and the total duration of concurrent and adjuvant chemotherapy with temozolomide was 26.7 weeks. Twenty-six and thirteen patients received corticosteroid therapy during CCRT and adjuvant chemotherapy, respectively.
Survival and progression
Of the 73 patients with available response data, 20 patients (27%) demonstrated complete response, 33 patients (45%) had partial response, 14 patients (19%) had stable disease, 6 patients (8%) had progressive disease as a best response. The objective response rate (complete and partial response) was 73%, and the tumor control rate (complete and partial response and stable disease) was 92%. At the time of analysis, 67 patients (65%) had died, and the median follow-up time was 17 months (range, 1-79 months).
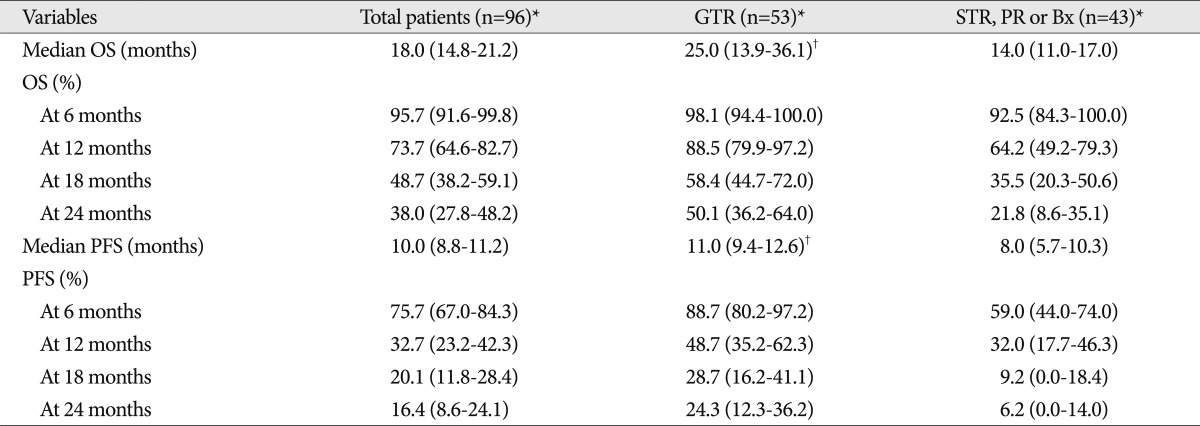
The Kaplan-Meier analyses were performed with the 96 patients who completed the CCRT. The median OS of these patients was 18 months [95% confidence interval (CI), 14.8-21.2] and the 6-, 12-, 18-, and 24-month OS were 95.7, 73.7, 48.7 and 38.0%, respectively. The median PFS was 10.0 months (95% CI : 8.8-11.2) and the 6-, 12-, 18-, and 24-month PFS were 75.7, 32.7, 20.1, and 16.4%, respectively. The PFS and OS values are summarized in Table 3, and the Kaplan-Meier survival curves of the patients with newly diagnosed glioblastomas are illustrated in Fig. 1.

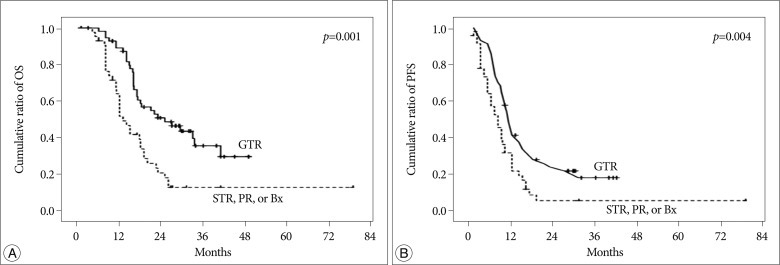
Kaplan-Meier estimates of (A) overall survival (OS) and (B) progression-free survival (PFS) in 96 patients suffering from newly diagnosed glioblastoma who were treated with temozolomide during and after radiotherapy.
Prognostic factors, including the age at diagnosis, sex, tumor location, preoperative KPS, postoperative KPS, extent of surgical resection and MGMT promoter methylation were analyzed in these patients. Among these factors, the only significant OS and PFS prognostic factor was the extent of surgical resection. The median OS and PFS of the GTR group (25.0 and 11.0 months, respectively) were longer than those of the STR, PR and biopsy groups (14.0 and 8.0 months, respectively); this difference was significant (hazard ratio, 2.277; 95% CI, 1.381-3.754; p=0.001 and hazard ratio, 1.931; 95% CI, 1.234-3.023; p=0.004, respectively). The PFS and OS values of these groups are summarized in Table 3, and the Kaplan-Meier survival curves are illustrated in Fig. 2.
Toxicity
The adverse events were analyzed separately by the period of CCRT and adjuvant therapy with temozolomide. During the CCRT with temozolomide treatment, 8 (8.1%) patients presented with grade 3 or 4 hematological toxicities, including 1 patient with leukopenia, 2 with neutropenia, 3 with lymphopenia, and 5 with anemia. Non-hematological toxic effects during the CCRT period tended to be grade 1 or 2. During the adjuvant therapy period, 16 (17.6%) patients presented with grade 3 or 4 hematological toxicities, including 6 patients leukopenia, 3 with neutropenia, 5 with lymphopenia, 7 with thrombocytopenia, and 3 with anemia. Non-hematological toxicity during the adjuvant therapy period was not available. All the patients that experienced toxicities from the chemotherapeutic agent received the delayed chemotherapy and tolerated the treatment well during therapy. No opportunistic infections were noted. The distribution of the CCRT and adjuvant therapy with temozolomide-related toxicities is shown in Table 4.
DISCUSSION
Only a few years ago, most patients who were diagnosed with primary glioblastoma died despite combination therapy including microsurgery, radiotherapy, and chemotherapy18). However, postoperative adjuvant treatments have evolved into a new era since the introduction of the chemotherapeutic drug temozolomide.
Temozolomide, which is a second-generation oral alkylating agent, performs antitumor activity by methylating the guanine residues of tumor cell DNA, thus creating a mismatch that an enzyme repair system cannot fix2,8,15). Temozolomide has demonstrated antitumor activity in the treatment of malignant gliomas and has been approved for the treatment of recurrent malignant glioma and newly diagnosed glioblastoma1,5,6,17,20). A randomized prospective study reported in 2005 showed that CCRT with temozolomide was more effective than radiotherapy alone in patients newly diagnosed and histopathologically confirmed to have a glioblastoma18). The patients in this study had a median OS of 14.6 months for CCRT with temozolomide and 12.1 months for radiotherapy alone. The 2-year survival rate was also higher for patients treated using CCRT with temozolomide (26.5%) than with radiotherapy alone (10.4%). At present, CCRT followed by adjuvant chemotherapy with temozolomide is widely accepted as a standard protocol for patients newly diagnosed with a glioblastoma. Since the Stupp report, most Korean institutions use this concurrent protocol with temozolomide for primary glioblastoma treatments. Some single center studies reported the outcome of CCRT followed by adjuvant chemotherapy with temozolomide for Korean patients using Stupp's regimen3,7,8,10,14).
However, there have been no reported multicenter trial data from Korea. The objective of this study was to report the current results of a prospective multicenter clinical trial investigating the use of CCRT with temozolomide in Korea and to determine a standard treatment protocol for Korean patients with a primary glioblastoma. A total of 103 patients from 4 institutes were enrolled in this study, and 93 and 54% of these patients completed the CCRT and adjuvant chemotherapy with temozolomide, respectively. These completion rates were comparable to the results of a previous well-designed globalized trial18).
Notably, the median PFS and OS of patients with glioblastoma treated with CCRT were 10.0 and 18.0 months respectively. This result seems to be slightly more favorable than Stupp's results and similar to that of a single center study in Korea 3,7,18). It is possible that the effect of temozolomide may be more favorable in Korean patients than in other patients. However, there has not been any evidence in support of this assumption exists, and more sophisticated studies should be designed to test this hypothesis.
The favorable outcome of the CCRT with TMZ in Korean patients may be due to the higher rate of patients with GTR in Korea than those in the Stupp's study. While 40% of the patients in Stupp's study underwent GTR, 55% of the patients in this study underwent GTR18). In effect, the only significant prognostic factor of OS and PFS was the extent of surgical resection in this study. Recent studies also reported that the extent of surgical resection was a significant prognostic factor for patients with a primary glioblastoma3,8,11,16). These data suggest that the more favorable survival rate in our study as compared with Stupp's study may be due to the higher GTR rate.
Unfortunately, this study revealed that other prognostic factors, including age, KPS score, and MGMT promoter methylation status, were not significant prognostic factors. This is probably because MGMT promoter methylation was investigated in such a small number of patients. To reach a definitive conclusion, future studies should include additional data regarding biological factors, such as methylation of the MGMT promoter, for all or most of the studied patients9).
The treatment protocol used in this study was already recognized as the standard protocol for newly diagnosed glioblastoma, and this issue is the limitation of this study. Moreover, this study lacks the control group and is not a randomized comparative study. However, this study was the first prospective multi-institutional study for the glioblastoma treatment in Korean patients. Therefore, the further randomized controlled study is also needed for improving the survival of Korean glioblastoma patients. The results of this study can become control data for this further trial.
CONCLUSION
The CCRT and adjuvant chemotherapy with temozolomide may prolong the survival of Korean patients with a glioblastoma and may be tolerable in terms of toxicity. The significance of this study is that our study results may help in determining a proper chemoradiotherapy regimen for newly diagnosed glioblastomas in Korea by proving the effectiveness and safety of Stupp's regimen in Korean patients. The results of this study are also expected to serve as control data for further randomized comparative trials for patients with a glioblastoma in Korea.