Effect of Cisternal Drainage on the Shunt Dependency Following Aneurysmal Subarachnoid Hemorrhage
Article information
Abstract
Objective
Shunt-dependent chronic hydrocephalus (SDCH) is known to be a major complication associated with aneurysmal subarachnoid hemorrhage (aSAH). Old age is known to be one of numerous factors related to the development of SDCH. This study investigated whether postoperative cisternal drainage affects the incidence of SDCH and clinical outcome in elderly patients with aSAH.
Methods
Fifty-nine patients participated in this study. All patients underwent aneurysmal clipping with cisternal cerebrospinal fluid (CSF) drainage. Clinical variables relevant to the study included age, sex, location of ruptured aneurysm, CT finding and clinical state on admission, clinical outcome, and CSF drainage. We first divided patients into two groups according to age (<70 years of age and ≥70 years of age) and compared the two groups. Secondly, we analyzed variables to find factors associated with SDCH in both groups (<70 years of age and ≥70 years of age).
Results
Of 59 patients, SDCH was observed in 20 patients (33.9 %), who underwent shunt placement for treatment of hydrocephalus. Forty seven percent of cases of acute hydrocephalus developed SDCH. In the elderly group (≥70 years of age), the duration and amount of CSF drainage did not affect the development of chronic hydrocephalus.
Conclusion
In elderly patients, although the incidence of SDCH was significantly higher, clinical outcome was acceptable. The duration and the amount of cisternal drainage did not seem to be related to subsequent development of chronic hydrocephalus within elderly patients aged 70 or older.
INTRODUCTION
Shunt-dependent chronic hydrocephalus (SDCH) is a well-known sequelae of aneurysmal subarachnoid hemorrhage (aSAH). The incidence of SDCH in patients of all ages after aSAH ranges from 10 to 48 %4,12,32,36,38). Numerous factors have been reported as being associated with the occurrence of chronic hydrocephalus after aSAH such as increasing age, hypertension, intraventricular hemorrhage (IVH), diffuse subarachnoid blood, posterior circulation aneurysm sites, focal ischemic deficits, large ventricles at the time of admission, poor Hunt-Hess and Fisher grades, symptomatic vasospasm, aneurysmal rebleeding, and being of the female sex1,29,30,32,36).
Because aging individuals are comprising and increasingly larger percentage of the general population, aSAH in elderly patients is becoming an issue of greater importance, and the number of surgical procedures or intervention procedures performed in elderly patients with aSAH is increasing. Although coil embolization has been preferentially performed to date, elderly patients previously considered inoperable are now undergoing operations with acceptable rates of morbidity and mortality5,10,11,17). SDCH after aSAH develops frequently in elderly patients. To improve the outcome in elderly patients with aSAH, an understanding of the pathophysiology of hydrocephalus after aSAH is very important.
Several mechanisms have been reported to explain the development of hydrocephalus among patients after aSAH. In some studies, aSAH appears to cause fibrosis of the leptomeninges and arachnoid granulation, leading to alterations in cerebrospinal fluid (CSF) dynamics, impaired CSF absorption, and chronic hydrocephalus1-3). Acute hydrocephalus may occur via obstructive mechanisms when blood products or adhesions block CSF circulation within the ventricular system7,37).
Cisternal drainange may remove remaining blood clots and improve CSF dynamics. Improved CSF flow may reduce subarachnoid fibrosis and vascular inflammation, thereby leading to decreased hydrocephalus. So, we performed a retrospective study on whether postoperative cisternal drainage affects the incidence of SDCH and clinical outcome in elderly patients with aSAH.
MATERIALS AND METHODS
Patient population
This study was based on a series of 179 patients consecutively diagnosed with aSAH at our hospital between January 2007 and November 2010. On admission, each patient's clinical condition was graded according to the Hunt-Hess grading scale and a computed tomography scan of each incidence of aSAH classified according to the Fisher grading system. Inclusion criteria were as follows : 1) patients with SAH due to anterior circulation aneurysm rupture and a Fisher grade of 3 or 4, 2) patients who underwent direct aneurysm clipping with cisternal drainage within 72 hours (Days 0-2) after ictus, 3) patients older than 18 years of age, 4) clinical Hunt-Hess grade of III or IV, and 5) patients without preoperative rebleeding or grave systemic complications. According to these criteria, 59 patients were included in the study. 120 patients were excluded due to posterior circulation (25), coiling (37), a Fisher grade of 2 (38), a Hunt-Hess grade of V (13), and preoperative external ventricular drainage (7). Acute hydrocephalus is defined as Evan's ratio >0.3 on brain CT scan at admission. SDCH is defined when requiring a shunt procedure.
Surgical procedures
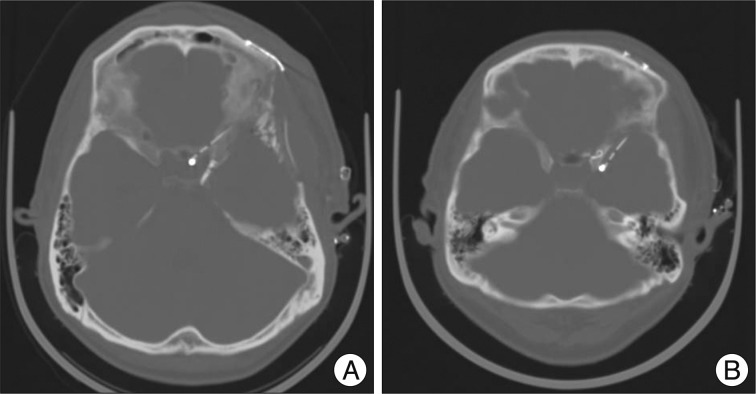
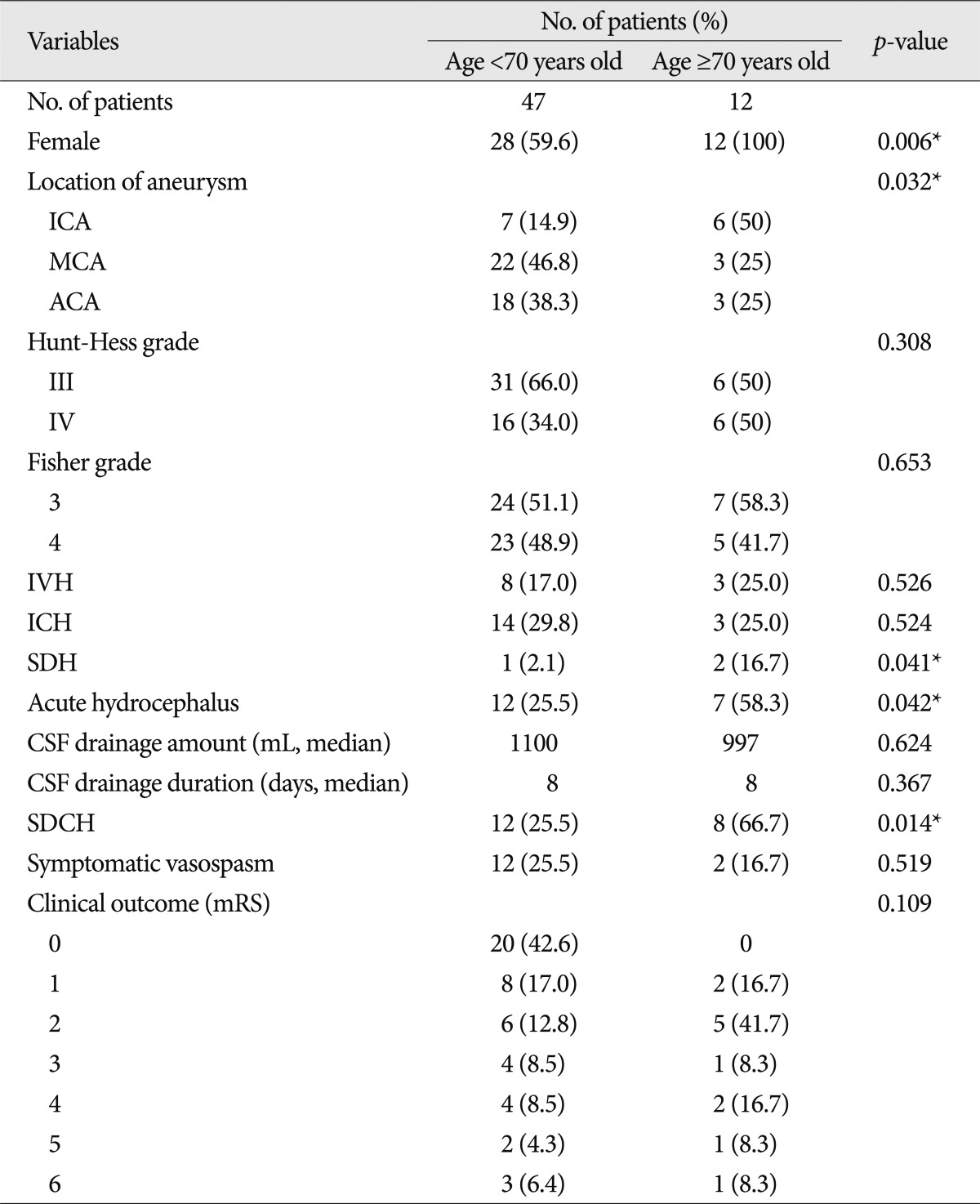
After the aneurysm was clipped, removal of the remaining subarachnoid clot in the basal cistern and surrounding the M1 and a part of the M2 portion of the middle cerebral artery was performed. The prepontine clot was aspirated when possible after opening Liliequist's membrane. After the removal of the subarachnoid hematoma, a cisternal drainage tube was placed in the chiasmatic cistern or carotid cistern (Fig. 1). The opening pressure was set to 10 cm H2O from the external auditory meatus in the supine position and continuous drainage of CSF was performed. A cisternal drainage tube was removed on the 7th postoperative day or when it ceased to function due to obstruction. If necessary, lumbar drainage was performed. Daily drainage amount and drainage duration were measured and recorded.
Management protocol
All patients received standard pre- and post-operative management. All patients received CT angiography within the first 48 hours after aneurysm treatment to identify procedural complications and drainage tube location. Then, postoperative CT scans were performed every week before discharge or when/if the patient's neurological condition worsened. Transcranial doppler tests were performed every other day between day 4 and day 14 after aSAH and checked daily if a mean velocity in any insonated vessel was more than 150 cm/sec or if the patient exhibited clinical deterioration. When symptomatic cerebral vasospasm was suspected, we elevated mean blood pressure more than 20 % with the use of an inotropic agent and volume expander. Cerebral angiography was performed if neurologic state did not improve and, if needed, chemical or mechanical angioplasty was performed.
Clinical data
The clinical variables investigated were 1) age, 2) sex, 3) Fisher grade at admission, 4) clinical Hunt-Hess grade at admission, 5) presence of IVH, intracerebral hemorrhage (ICH), and subdural hematoma (SDH), 6) presence of hydrocephalus at admission, 7) the location of ruptured aneurysm, 8) CSF drainage amount and duration, 9) presence of symptomatic vasospasm, 10) SDCH, and 11) clinical outcome. The modified Rankin Scale (mRS) was used to describe the clinical outcome 3 months after occurrence of initial bleeding.
Statistical analysis
At first, we divided the study participants into two groups according to age (<70 years, ≥70 years) and compared the two groups. Secondly, we analyzed variables to find factors associated with SDCH in both groups (<70 years of age and ≥70 years of age). Statistical analysis was performed using PASW statistics 18.0 (IBM, Armonk, NY, USA). Data are presented as the median value, and statistical significance was defined as p<0.05 for all comparisons. Statistical significance was analyzed with the chi-square test or Fisher exact for categorical variables and the independent-Samples Mann-Whitney U test for continuous variables. Multivariate analysis was not performed due to small sample size.
RESULTS
Fifty nine patients were studied. Patient ages ranged from 32 to 82 years. The study population consisted of 19 male (32.2%) and 40 female patients (67.8%). A ruptured aneurysm was located on the anterior cerebral artery (ACA) in 21 patients (35.6%), the internal carotid artery (ICA) in 13 patients (22.0%), and the middle cerebral artery (MCA) in 25 patients (42.4%). According to preoperative clinical grading, 37 patients received a Hunt-Hess grade of III (62.7%) and 22 a Hunt-Hess grade of IV (37.3%). There were 31 patients with a Fisher grade of 3 (52.5%) and 26 patients with a Fisher grade of 4 (47.5%). Initial brain CT scan showed IVH in 11 patients (18.6%), ICH in 17 patients (28.8%), SDH in 3 patients (5.1%), and acute hydrocephalus in 19 patients (32.2%).
Two patients with postoperative cisternal CSF drainage suffered meningitis, which was successfully treated with antibiotic administration. The median duration of external CSF drainage in patients including cisternal and lumbar CSF drainage was 8 days. The median amount of CSF drained was 1011 mL, and 14 patients underwent external CSF drainage for over 10 days. SDCH was observed in 20 patients (33.9%) who then underwent shunt placement for treatment of hydrocephalus. Forty seven percent of cases of acute hydrocephalus progressed to SDCH. Symptomatic vasospasm was identified in 14 patients (23.7%). Symptoms due to vasospasm were transient in all patients.
Comparison between the two age groups
Table 1 shows comparison of the two age groups. There were no significant differences between the two groups in patient characteristics (Hunt-Hess grade, Fisher grade, the presence of IVH and ICH, and the incidence of symptomatic vasospasm) or CSF drainage (amount and duration). In the elderly group (≥70 years of age), the presence of acute hydrocephalus (p=0.042) and SDH (p=0.041), and the incidence of SDCH (p=0.014) were significantly higher. All patients in the elderly group were female. Location of ruptured aneurysm between the two groups was significantly different (p=0.032).
Factors related to shunt dependency within younger group (<70 years of age)
Results of univariate analysis are summarized in Table 2. There were marginally significant differences in cisternal CSF drainage amount and duration between patients with SDCH and without SDCH (p=0.093 and p=0.078, respectively). The other factors showed no significant difference between two groups.
Factors related to shunt dependency within elderly group (≥70 years of age)
The sex variable was excluded because all patients in the elderly group (≥70 years of age) were female. Results of univariate analysis are summarized in Table 3. There was a significant difference only in location of ruptured aneurysm between patients with SDCH and without SDCH.
DISCUSSION
SDCH is a common complication after aSAH. Hydrocephalus after aSAH is classified according to the time of onset : 1) acute (0-3 days after aSAH), 2) subacute (4-13 days after aSAH), and 3) chronic hydrocephalus (≥14 days after aSAH). Although the precise mechanism leading to development of hydrocephalus after aSAH is not fully understood, acute hydrocephalus following aSAH is thought to result from blockage of CSF flow. Intraventricular clots and the high viscosity of the CSF block the ventricular foramina, inducing more resistance to CSF flow and increased ventricular flow2,7,33,34). Also, the blood in the basal cistern and ventricle system increase CSF resistance, creating a pressure gradient and inducing hydrocephalus37). The occurrence of aSAH can also cause fibrosis of leptomeninges and arachnoid granulation16, 35), which leads to diminished CSF absorption and chronic hydrocephalus requiring shunt treatment1-3,6,28,35). Another hypothesis proposes that chronic hydrocephalus occurs due to fibrin and protein products in the blood in the arachnoid villi inhibiting absorption of CSF16,20).
Age is thought to be a potential factor of chronic hydrocephalus following aSAH12,17,32,38). In the elderly group in this study, SDCH occurred in 8 patients (66.7%). Numerous theories have been reported to explain the relationship between occurrence of chronic hydrocephalus and elderly patients. Due to wider subarachnoid spaces, older patients can hold larger amounts of subarachnoid blood, thus increasing their risk of developing CSF circulation disturbances40). Also, with age, the extent of meningeal fibrosis increases, leading to impaired CSF circulation and decreased CSF absorption16,35). Some investigators have suggested the importance of the minor CSF pathway, namely CSF absorption by the capillaries of the brain via the extracellular space within the parenchyma, as a potential factor leading to the occurrence of normal pressure hydrocephalus32). Chronic hydrocephalus can more frequently occur in elderly patients due to disturbance of the minor pathway within the parenchyma because patients of advanced age with aSAH are susceptible to cerebral ischemia. Others have postulated that the CSF circulation time is prolonged in elderly patients secondary to decreased CSF secretion, resulting in relative CSF stagnation21).
Generally, acute hydrocephalus ranges from 15 to 31% of patients having experienced aSAH8,22,23,31,38). The presence of acute hydrocephalus, one of possible risk factors of SDCH after aSAH, was observed in 19 patients (33.9%) in this study, a higher percentage than that reported in current literature, perhaps due to the inclusion criteria of this study, specifically a Fisher grade of 3 or 4. Forty seven percent of patients with acute hydrocephalus developed SDCH. After comparing the two age groups, the presence of acute hydrocephalus in the elderly group (≥70 years of age) was found to be significantly higher than in the younger group (<70 years of age). In the elderly group, acute hydrocephalus developed in 7 patients and progressed to SDCH in all except 1 patient (86%).
As previously mentioned, the effect of IVH on the development of acute hydrocephalus may be that IVH leads to an obstructive form of hydrocephalus2,7,33,34). In this study, 36% of patients with IVH developed SDCH. Between the two age groups, there was no significant difference in the presence of IVH.
In this study, there was a significant difference in the location of the ruptured aneurysm between the two age groups. In the elderly group, a ruptured ICA aneurysm occurred with greater frequency than ACA or MCA aneurysms. In 25 patients with a ruptured MCA aneurysm, IVH was not seen. There was acute hydrocephalus in 3 of 25 patients and chronic hydrocephalus in 1 of 25 patients. Various studies have reported a potential relationship between the location of the ruptured aneurysm and the development of hydrocephalus. Many authors reported that acute hydrocephalus develops less frequently in patients with an MCA aneurysm than in those with ACA, ICA or posterior circulation aneurysms25,26,40). The low incidence rate in patients with MCA aneurysms may be explained by the fact that subarachnoid blood clots are relatively scarce in the contralateral sylvian fissure and interhemispheric fissures, therefore, the CSF circulation pathway in these areas is spared from possible obstruction to flow26,40).
Cerebral vasospasm following aSAH constitutes one of the most serious complications affecting the prognosis of patients. It has been recommended that the removal of subarachnoid blood clots along the cerebral arteries can reduce cerebral vasospasm, but it is impossible to remove all blood clots. However, cisternal or lumbar drainage has been reported as a useful procedure to remove bloody CSF and to improve the prognosis through prophylaxis and relief of vasospasm12-15). The effects of cisternal drainage for the development of SDCH are not clear. Some authors reported that the need for shunt operation was greater in patients with cisternal drainage, especially in those with high-volume drainage or cisternal drainage which seemed to exacerbate subarachnoid fibrosis and impair CSF absorption9,18,24,27). Conversely, some authors reported that early aneurysmal surgery and washout of bloody CSF lessened the risk of subsequent subarachnoid fibrosis and impaired CSF outflow19,39). In this study, cisternal drainage was used in all patients, being expected to eliminate residual subarachnoid clots and spasmogens in the CSF from the cistern and promote CSF circulation. In univariate analysis of factors related to shunt dependency within younger group (Table 2), SDCH was related to the longer duration and the more amount of CSF drainage with marginal significance, but in elderly group (Table 3), The duration and the amount of cisternal drainage was not related to SDCH.
Elderly patients (≥70 years of age) have been reported to show a worse outcome than younger patients because of poor recovery from primary brain damage, more severe cerebral ischemia due to vascular narrowing, frequent systemic complications, and low cerebral compliance10,11,17). However, there was no significant difference in clinical outcome between the two age groups in this study. Although no one patient recovered fully, 8 patients showed favorable outcomes. The mRS score of 3 indicates moderate disability with the patient requiring some help but having the ability to walk unassisted.
The limitations of this study were the small sample size and a selected population who received cisternal drainage. Multivariate analysis could not be performed to determine the effect of each variable on the development of SDCH. In this study, we excluded the patients with a good clinical state (Hunt-Hess grade of I or II), dismal state (Hunt-Hess grade of V), or mild aSAH (Fisher grade of 1 or 2) to decrease confounding factors.
CONCLUSION
In univariate analysis of factors related to shunt dependency within younger group, SDCH was related to the longer duration and the more amount of CSF drainage with marginal significance. In elderly patients, although the incidence of SDCH was significantly higher, clinical outcome was acceptable. The duration and the amount of cisternal drainage did not seem to be related to chronic hydrocephalus in elderly patients. SDCH occurred more frequently in elderly patients with ACA or ICA aSAH. We speculated that acute hydrocephalus and IVH, accompanied with ACA or ICA aSAH, affected the rate of occurrence of chronic hydrocephalus.