Spontaneous Cerebellar Hemorrhage with the Fourth Ventricular Hemorrhage : Risk Factors Associated with Ventriculoperitoneal Shunt
Article information
Abstract
Objective
The purposes of this study are to investigate the factors that may be related to ventriculoperitoneal (VP) shunt in patients with cerebellar hematoma and the effect of severe fourth ventricular hemorrhage, causing obstructive hydrocephalus on subsequent VP shunt performance.
Methods
This study included 31 patients with spontaneous cerebellar hematoma and concomitant fourth ventricular hemorrhage, who did not undergo a surgical evacuation of hematoma. We divided this population into two groups; the VP shunt group, and the non-VP shunt group. The demographic data, radiologic findings, and clinical factors were compared in each group. The location of the hematoma (whether occupying the cerebellar hemisphere or the vermis) and the degree of the fourth ventricular obstruction were graded respectively. The intraventricular hemorrhage (IVH) score was used to assess the IVH severity.
Results
Ten out of 31 patients underwent VP shunt operations. The midline location of cerebellar hematoma, the grade of fourth ventricle obstruction, and IVH severity were significantly correlated with that of VP shunt operation (p=0.015, p=0.013, p=0.028). The significant variables into a logistic regression multivariate model resulted in statistical significance for the location of cerebellar hemorrhage [p=0.05; odds ratio (OR), 8.18; 95% confidence interval (CI), 1.00 to 67.0], the grade of fourth ventricle obstruction (p=0.044; OR, 19.26; 95% CI, 1.07 to 346.6).
Conclusion
The location of the cerebellar hematoma on CT scans and the degree of fourth ventricle obstruction by IVH were useful signs for the selection of VP shunt operation in patients with spontaneous cerebellar hematoma and concomitant acute hydrocephalus.
INTRODUCTION
Spontaneous cerebellar hematoma represents 5 to 13% of all cases of spontaneous intracranial hemorrhage5,8). One of the symptoms of cerebellar hematoma is acute hydrocephalus, which generally results from the fourth ventricular compression by severe cerebellar hematoma; however, it can easily be treated effectively by surgical evacuation2,3,14). Obstructive hydrocephalus can also occur by severe fourth ventricular hemorrhage in a small number of cerebellar hematomas, not requiring surgical treatment. In such case, permanent cerebrospinal fluid (CSF) diversion may be required due to the development of chronic hydrocephalus1,18). Several studies have investigated the relationship between the intraventricular hemorrhage (IVH) severity and ventriculopoeritoneal (VP) shunt in cerebellar hemorrhage. They have also reported that severe IVH must have an effect on ventricular dysfunction, for a VP shunt to be necessary6-9,12,13,15). However, studies that deal with the role of fourth ventricular hemorrhage, causing obstructive hydrocephalus, and the relation to VP shunt performance have seldom been reported.
Thus, authors investigated the factors that are related to VP shunt in patients with cerebellar hematoma and concomitant fourth ventricular hemorrhage. In addition, the authors also investigated the effect of severe fourth ventricular hemorrhage, which causes obstructive hydrocephalus on subsequent VP shunt performance.
MATERIALS AND METHODS
Patient population and study design
Between January 2004 and December 2011, 37 patients with spontaneous cerebellar hemorrhage and concomitant fourth ventricular hemorrhage were admitted to the neurosurgical unit of our institution. Cases with traumatic causes or with an underlying neoplasm, aneurysm, or evident arteriovenous malformation were excluded from this analysis. Six of the 37 patients underwent a surgical evacuation of the cerebellar hematoma. The surgical criteria in patients with cerebellar hematomas were as follows : 1) large (>3 cm) cerebellar hemorrhages, 2) large cerebellar hemorrhage with hydrocephalus and/or direct brainstem compression by the hematoma and surrounding swelling, or both, and 3) the depressed level of consciousness2,3).
The sample in this study consisted of 31 patients who did not receive surgical treatment, and were managed with ventricular drainage only or conservative treatment. We divided this population into two groups; the VP shunt group (group A) and the non-VP shunt group (group B). We compared the demographic data, radiologic findings, and clinical factors in each group. These various features were evaluated on the initial CT scans after presentation. The location of the hematoma (whether occupying the cerebellar hemisphere or the vermis) was noted and graded. Patients who had cerebellar hemorrhage with fourth ventricular hemorrhage were classified into 2 grades, according to the degree of fourth ventricle obstruction by IVH, as follows : Grade I, partially obstructed, CSF is still visible in the fourth ventricle; and Grade II, complete obliteration, with anterior shift distorting the brainstem (Fig. 1). The diagnosis of acute hydrocephalus was made on the basis of CT criteria, using the relative bicaudate index (RBCI) (width of the frontal horns at the level of the caudate nuclei divided by the width of the parenchyma at the same level >95th percentile for age). The RBCI >1 meant the presence of hydrocephalus10,20).
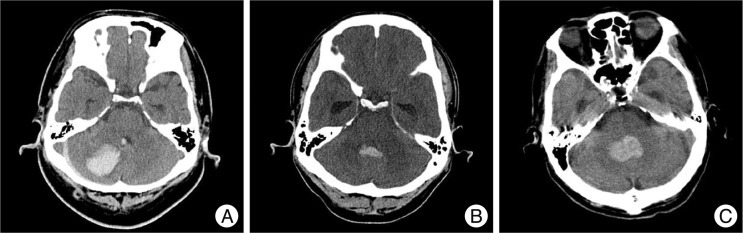
CT scans demonstrating different cerebellar hematomas and the degree of the fourth ventricle obstruction on the axial CT slice. A : hematoma in one cerebellar hemisphere and partial obstruction of fourth ventricle (Grade I). B : hematoma occupying midline or vermis and partial obstruction of fourth ventricle (Grade I). C : hematoma involving both cerebellar hemisphere and vermis and complete obstruction of fourth ventricle (Grade II).
The IVH severity was assessed by determining the IVH score. The IVH score grades each lateral ventricle with a score of 0 (no blood or small amount of layering), 1 (up to one third filled with blood), 2 (one to two thirds filled with blood), and 3 (mostly or completely filled with blood). The third and fourth ventricles received a score of 0 for no blood, or 1 if they were partially or completely filled with blood. Hydrocephalus was coded as present (1) or absent (0). The IVH score was calculated using the following equation : 3×(right lateral ventricle score+left lateral ventricle score+hydrocephalus score)+third ventricle score+fourth ventricle score. IVH score ranges from 0 (no IVH) to 23 (all ventricles filled with blood and hydrocephalus)4,6). The size of the hematoma was assessed by calculating the maximal transverse diameter and the volume. The volume of hematoma was measured by calculating the area occupied by the hyperdense hematoma on each slice of the CT image and multiplying the area by the thickness of each slice.
Sixteen patients with acute hydrocephalus had been treated by an external ventricular drainage (EVD). We performed VP shunt operation in the following EVD weaning failure7); 1) the EVD had to be reopened because of increased intracranial pressure or clinical deterioration; 2) a CSF leak developed during the EVD weaning process; and 3) a CT scan obtained 24 hours after drain closure revealed hydrocephalus. If weaning failed, 1 or more criteria were met, and the VP shunt operation was performed.
Six patients with IVH had received injection of urokinase (25000 international unit) in 1 mL of normal saline solution, every 12 hours. The first intraventricular catheter (IVC) injection occurred from 12 hours to 24 hours after the initial bleeding episode. The IVC injections were performed with standard sterile technique. Injections were preceded by gentle aspiration of no more than 5 mL of cerebrospinal fluid. Injection of the urokinase was followed by a 3 mL flush with normal saline solution. Intracranial pressure and cerebral perfusion pressure were monitored before, during, and after the injections. After 1 hour of closure, the IVC was reopened. Study agent injections continued every 12 hours. The resolution rate was estimated by clot half-life (the mean time to achieve a clot 50% of its original size)15,16).
Statistical analysis
The data was analyzed using SAS 9.12 (Institute Inc., Cary, NC, USA) for Windows. Independent Student's t-test, chi-square test and the Fisher exact probability test were used for statistical comparisons between the two groups. Univariate and multivariable logistic regressions were calculated to identify independent predictors of VP shunt performance. In addition, a receiver operating characteristic (ROC) curve analysis was used to determine the predictive values of IVH score, with regard to VP shunt performance. The results were considered significant for probability values less than 0.05.
RESULTS
Patient characteristics
Of the total 31 eligible patients (11 men and 20 women) with the mean age of 57.8 years (range, 18-84 years), 10 of them received a VP shunt (group A) and 21 received a non-VP shunt (group B). Table 1 shows the results of the present study. The presenting Glasgow Coma Scale (GCS) score in the consciousness levels on arrival at our neurosurgical center was found. An initial GCS score of less than 9 was in 5 cases (group A : 2, group B : 3) and more than 9 was in 26 cases (group A : 8, group B : 18). The hematoma was confined to one cerebellar hemisphere in 10 cases (group A : 0, group B : 10), in a midline or vermian location in 12 cases (group A : 5, group B : 7), and involved the hemisphere and vermis in 9 cases (group A : 5, group B : 4). The fourth ventricle was partially obstructed (Grade I) in 13 case (group A : 1, group B : 12), and completely obliterated (Grade II) in 18 cases (group A : 9, group B : 9). Obstructive hydrocephalus was identified in 26 cases (group A : 10, group B : 16), who had RBCI >1.0, based upon a CT scan on admission. The mean IVH score was 7.6 (range : 0-23, group A : 11.1±7.0, group B : 6.0±6.1). The mean maximal transverse diameter of the hematoma was 2.6 cm (range : 1.3-4.3 cm, group A : 2.8±0.9, group B : 2.5±0.8). The mean volume of cerebellar hematoma was 5.9 mL (range : 1-31 mL, group A : 7.4±4.5, group B : 5.2±4.5). Sixteen patients with acute hydrocephalus were treated by EVD. They all did not show an upward transtentorial herniation after EVD. Ten of them underwent VP shunt operation. The mean interval from EVD to VP shunt in group A was 8.5 days, and the mean duration of EVD patency in group B was 4.8 days. EVD-related infection occurred in only 2 (1.2%) patients from group A. Six of the 23 patients with IVH received intraventricular thrombolysis, using urokinase. Of them, 4 cases underwent VP shunt operation. The mean duration of clot half-life was 2.5 days (group A : 3.0 days, group B : 1.5 days). Location of cerebellar hemorrhage, the grade of fourth ventricle obstruction, and IVH score between the two groups were significantly different (p=0.015, p=0.013, p=0.028).
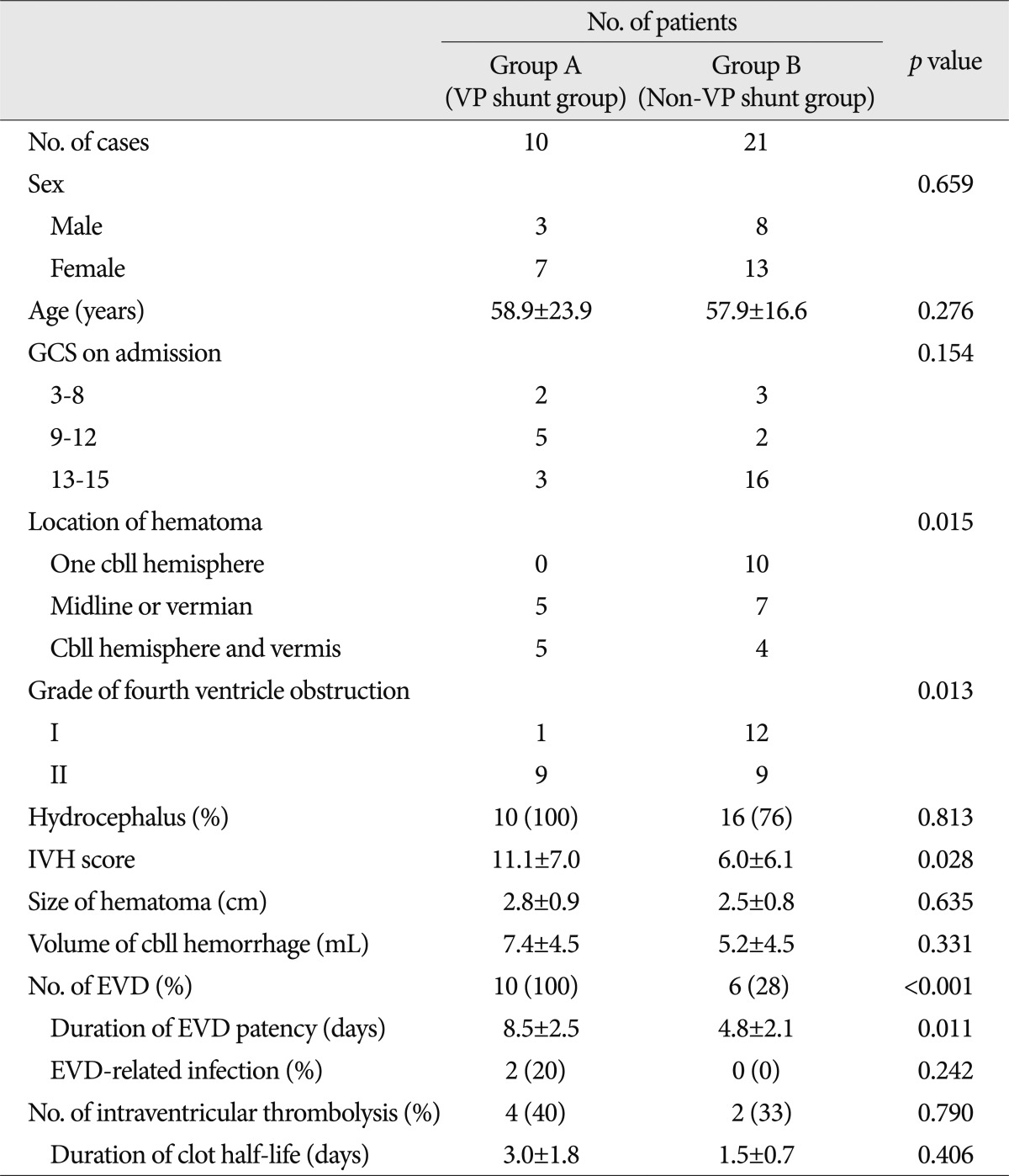
Clinical features in 31 patients with cerebellar hemorrhage and concomitant fourth ventricle hemorrhage for group A (VP shunt) and group B (non-VP shunt)
Stepwise introduction of the significant variables into a logistic regression multivariate model resulted in statistical significance for the location of cerebellar hemorrhage [p=0.05; odds ratio (OR), 8.18; 95% confidence interval (CI), 1.00 to 67.0], the grade of fourth ventricle obstruction (p=0.044; OR, 19.26; 95% CI, 1.07 to 346.6) (Table 2). In addition, ROC curve analysis showed a cutoff value of 6.5 in the IVH scores, which achieved 70.0% specificity and 76.2% sensitivity (Table 3). However, two patients who underwent VP shunt were all 4 (<6.5) of the IVH score in an initial CT scan; all of them had complete obliteration (Grade II) of only the fourth ventricle.
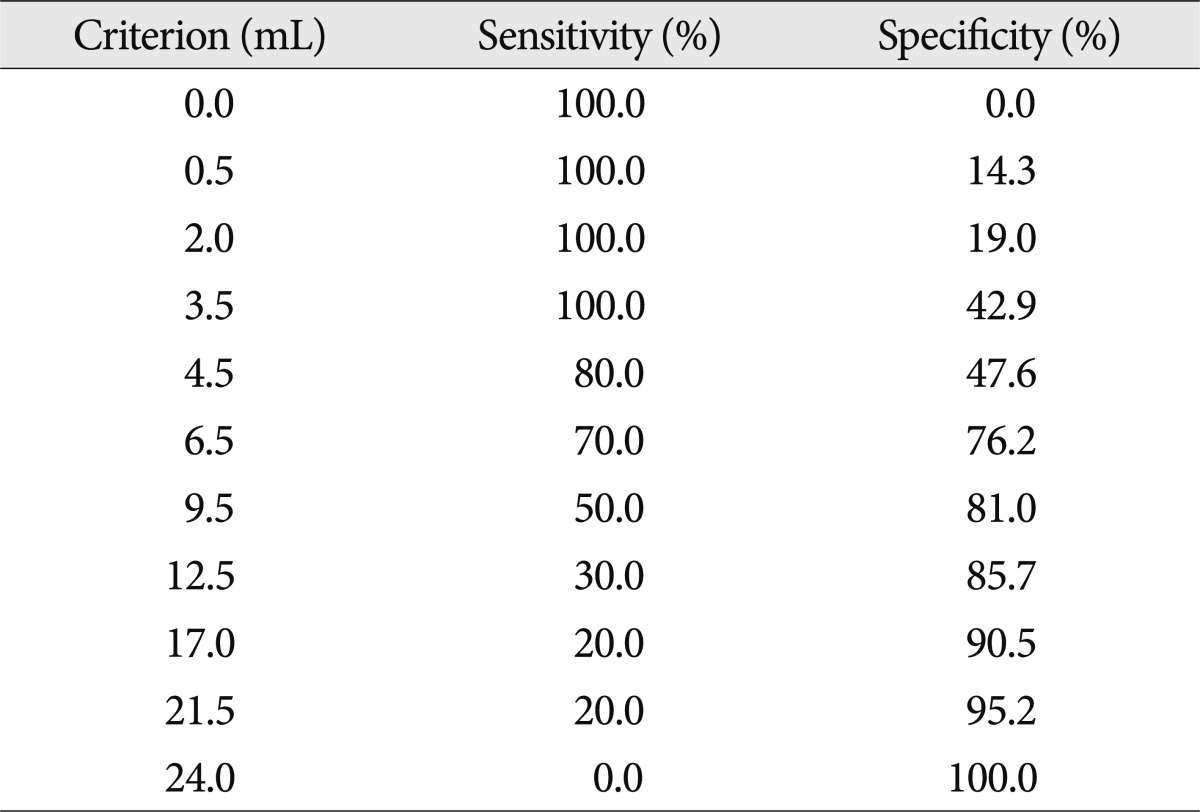
Criterion values and coordinates of an ROC curve as regard the IVH scores for cerebellar hemorrhage patients with IVH
The excluded 6 patients underwent a surgical evacuation of the cerebellar hematoma. All 6 cases had not received a VP shunt operation. The mean age was 64.3±9.0 years, and all had cerebellar hematoma involving the hemisphere and vermis. The fourth ventricle was partially obstructed in 2 cases, and completely obliterated in 4 cases. EVD was performed in 4 cases having acute hydrocephalus. The mean maximal transverse diameter of the hematoma was 4.6±0.4 cm (range : 4.1-5.3 cm). The mean volume of cerebellar hematoma was 35.3±8.7 mL (range : 27-47 mL). The mean admission duration, after a surgical evacuation of the cerebellar hematoma, was 23.5±5.9 days.
DISCUSSION
The existence of hydrocephalus and the development of chronic hydrocephalus is a problem, awaiting a solution in patients who didn't undergo surgical treatment for cerebellar hemorrhage. This may be affected by the location of a hematoma, the degree of IVH severity, and the grade of fourth ventricular obliteration. The importance of the location, especially the hematoma in the midline occupying the vermis, is related to the grade of fourth ventricular compression. In these cases, hematoma has ruptured into the fourth ventricle, and brought out greater degrees of fourth ventricular obstruction. Also, the hematoma in the vermis, without IVH, can result in hydorcephalus by fourth ventricle compression. Thus, midline hematomas may be associated with obstructive hydrocephalus, caused by fourth ventricle compression and IVH. In our series, the hematoma, occupying the vermis, tended to have the development of chronic hydrocephalus, and there was a significant difference in the location of cerebellar hematoma between the VP shunt group and the non-VP shunt group (p=0.015).
Many previous reports have indicated that IVH could be the most consistent predictor of the development of shunt-dependent hydrocephalus7,19). The lower threshold for a poor outcome at postadmission may be due to the prolonged exposure of the ventricles to the blood, which worsens IVH-mediated injuries and neurological outcome4,15). However, it is not clear how much IVH may affect a clinically ventricular dysfunction. Hwang et al.6) reported that the admission IVH volume of 6.0 mL was associated with a significant increase in the likelihood of poor functional outcome and subacute worsening in IVH volume, which may not have a significant influence on the outcome in patients with cerebellar hematoma. In our study, the degree of IVH severity may have a significant influence on the ventricular dysfunction and the progress of shunt-dependent hydrocephalus, and there was the significant difference in the IVH severity between the VP shunt group and the non-VP shunt group (p=0.028). Also, based on our data, the ROC curve analysis showed a cutoff value of 6.5 in the IVH scores, which achieved 70.0% specificity and 76.2% sensitivity. From the formula for converting IVH score to IVH volume, 6.5 in the IVH score means 3.7 mL in the IVH volume4). However, this cutoff value is insufficient to be used clinically because of not only its low sensitivity and specificity but also a small number of subjects. Additionally, two patients who underwent VP shunt received all 4 (<6.5) of IVH score in an initial CT scan; all of them had complete obliteration (Grade II) of only the fourth ventricle. This suggests that IVH score does not reflect the severity of IVH well and tends to overlook the important influence of fourth ventricular hemorrhage on obstructive hydrocephalus to be a necessity of a VP shunt. This is the reason why the fourth ventricle score was set at only 0 or 1 in the formula for calculating the IVH score. It means that not only IVH severity, but also fourth ventricular obstruction, should be estimated for a VP shunt operation.
EVD in patients having acute hydrocephalus with cerebellar hematoma can present an upward transtentorial herniation, causing neurologic deterioration11,17). Based on our data, 16 patients with acute hydrocephalus were treated by EVD. They all did not show an upward transtentorial herniation after EVD, this suggest that they have small amounts of cerebellar hematoma, which was not included in the aforesaid surgical criteria.
This study has some limitations. This study is retrospective; thus, selection bias and protocol deviations were inevitably present. That is, some patients who suffered cerebellar hematoma may not have been found, especially in the clinically severe cases. In addition, due to this study having a relatively small number of subjects, it lacks sufficient power to clarify clinical factors of shunt placement in patients with cerebellar hematoma and/or IVH. Finally, the location of hematoma, the degree of fourth ventricle obstruction, and hydrocephalus might be evaluated more by a magnetic resonance imaging (MRI) than CT. Therefore, a prospective, large sample sized trial, using an MRI, will be required.
CONCLUSION
Based on our data, the location of the cerebellar hematoma on CT scans and the degree of fourth ventricle obstruction by IVH were useful signs for the selection of VP shunt operation in patients with spontaneous cerebellar hematoma and concomitant acute hydrocephalus.