Im, Yoo, and Park: Proper Indication of Decompressive Craniectomy for the Patients with Massive Brain Edema after Intra-arterial Thrombectomy
Abstract
Objective
Numerous studies have indicated that early decompressive craniectomy (DC) for patients with major infarction can be life-saving and enhance neurological outcomes. However, most of these studies were conducted by neurologists before the advent of intra-arterial thrombectomy (IA-Tx). This study aims to determine whether neurological status significantly impacts the final clinical outcome of patients who underwent DC following IA-Tx in major infarction.
Methods
This analysis included 67 patients with major anterior circulation major infarction who underwent DC after IA-Tx, with or without intravenous tissue plasminogen activator. We retrospectively reviewed the medical records, radiological findings, and compared the neurological outcomes based on the “surgical time window” and neurological status at the time of surgery.
Results
For patients treated with DC following IA-Tx, a Glasgow coma scale (GCS) score of 7 was the lowest score correlated with a favorable outcome (p=0.013). Favorable outcomes were significantly associated with successful recanalization after IA-Tx (p=0.001) and perfusion/diffusion (P/D)-mismatch evident on magnetic resonance imaging performed immediately prior to IA-Tx (p=0.007). However, the surgical time window (within 36 hours, p=0.389; within 48 hours, p=0.283) did not correlate with neurological outcomes.
Conclusion
To date, early DC surgery after major infarction is crucial for patient outcomes. However, this study suggests that the indication for DC following IA-Tx should include neurological status (GCS ≤7), as some patients treated with early DC without considering the neurological status may undergo unnecessary surgery. Recanalization of the occluded vessel and P/D-mismatch are important for long-term neurological outcomes.
Key Words: Decompressive craniectomy · Mechanical thrombolysis · Intracranial pressure · Ischemic stroke · Cerebral infarction.
INTRODUCTION
The main target of the ischemic stroke treatment is the reperfusion of the ischemic penumbra tissue to salvage threatened but potentially viable brain tissues. Recanalization of an occluded artery within a limited time after the occlusion may allow the ischemic penumbra tissue to survive [ 1, 2, 4, 9, 16, 22, 34- 36]. Intravenous tissue plasminogen activator (IV-tPA) administration within a limited time from the symptom onset in patients without contraindications is regarded as a standard treatment for ischemic stroke patients treatment [ 32, 45]. Although IV-tPA administration is regarded as the first step of cerebral infarction treatment, it is difficult to achieve reperfusion of the larger vessel occlusions (LVO) such as internal cerebral artery terminus and proximal segment of the middle cerebral artery. Many recent papers have reported that the recanalization rates after IV-tPA are very low, and large hemispheric infarction (LHI) due to LVO occurs in up to 10% of all patients with ischemic strokes, with high mortality (~80%) and morbidity rates [ 6, 10, 14, 17, 19, 20, 30, 36, 39, 42]. The MR CLEAN (A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke) report found that the addition of intra-arterial thrombectomy (IA-Tx) improved neurological outcomes compared to those after IV-tPA alone. IA-Tx with or without IV-tPA is now widely performed, and the recanalization rate increased up to 80-90%. Thanks to the development of new endovascular devices and increasing clinician’s IA-Tx experience, the recanalization rate increased from approximately 70-80% to over 95% [ 5, 19, 29, 31, 48]. As the rigid skull encases the brain, major cerebral infarction can trigger increased intracranial pressure (ICP), which is associated with many hemodynamic problems [ 7, 17, 23, 46]. Decompressive craniectomy (DC) has been proposed as a therapeutic option for ICP unresponsive to conventional treatment modalities. DC preserves the cerebral blood flow (CBF) hemodynamics and prevents transtentorial herniation and secondary damage [ 11, 17, 37, 40, 47]. Many studies have reported that early DC improves survival rates and clinical outcomes compared to medical treatment alone. In some meta-analyses, surgical decompression within 24-48 hours of stroke onset doubled the chance of favorable functional outcomes. However, according to these reports, the outcomes were affected by patient age, the surgical time window, neuroradiological findings, and neurological status [ 10, 12, 18, 19, 27, 36, 39, 43, 44, 50]. However, those studies mainly focused on IV-tPA treatment and the indications for DC differently. More recently, several studies reported that DC is effective in patient survival after IA-Tx [ 15, 38]. DC surgery was vigorously researched in traumatic brain injury, and its guidelines recommend that DC be based on neurological status rather than the availability of a suitable surgical time window [ 25, 47]. This study aims to analyze whether the significance of the neurologic status should be included for DC indication to improve the patients’ clinical outcomes in patients following a major stroke with DC and IA-Tx.
MATERIALS AND METHODS
This retrospective observational study was approved by the Institutional Ethics Committee of Eunpyeong St. Mary’s Hospital and Uijeoungbu St. Mary’s Hospital, and the treatment protocol was approved by the Institutional Review Board of Eunpyeong St. Mary’s Hospital and Uijeoungbu St. Mary’s Hospital (approval number UC11RISI0187 and PC17RES10028) ( Fig. 1).
Patients
From March 2014 to October 2020, 323 patients underwent IA-Tx after IV-tPA, 96 of whom also underwent DC ( Fig. 1). Among these patients, 67 who presented with anterior circulation infarctions with valid data were analyzed in this study. Final neurological outcomes were determined according to the neurological status at the operation, perfusion/diffusion (PD)-mismatching (MR image taken within 3 hours from DC surgery), and surgical time window (time from stroke symptom onset to DC surgery). All patients underwent DC following IA-Tx with or without IV-tPA. We evaluated the neurological status as Glasgow coma scale (GCS) score just before the surgery and the clinical outcome by modified Rankin scale (mRS) three months after initial treatment. An mRS of 0-2 was regarded as a favorable outcome.
Neuroradiological evaluation
All patients underwent brain and neck computed tomography angiography (CTA) and brain computed tomography perfusion (Somatom Definition AS; Siemens Medical Systems, Munich, Germany). If a cut-off signal was evident in the suspected artery, the patient was diagnosed with LVO. Stroke protocol magnetic resonance imaging (MRI) was performed immediately after IV-tPA administration. T1-weighted sagittal scans, T2-weighted turbo-gradient spin echo/echo-planar diffusion-weighted imaging scans, and perfusion-weighted imaging (PWI) scans were obtained. Recanalization was defined as a flow that could be traced on a CTA image and was confirmed by follow-up cerebral angiography (Thrombolysis in cerebral infarction [TICI] grade more than 2b). P/D-mismatch was also evaluated by an acute stroke MRI tool after IV-tPA and just before the IA-Tx [ 41]. IA-Tx is recommended for non-recanalized patients after IV-tPA treatment. P/D mismatch is defined as a PWI lesion >100 mL in volume and ≥120% of the size of the diffusion lesion. In this study, the P/D mismatches were classified by a neuroradiologist not involved in stroke management. Patients who received IA-Tx therapy underwent follow-up computed tomography (CT) immediately and once more within 24 hours after IA-Tx. Increased density on the immediate CT image was defined as extravasation of the contrast medium, and increased density on both scans was defined as a hemorrhagic complication. A clinically significant, symptomatic intracranial hemorrhage was defined as neurological worsening of >4 points on the National Institutes of Health Stroke Scale/Score (NIHSS), attributable to a clot. Several randomized controlled studies defined symptomatic intracerebral hemorrhage as any type of intracerebral hemorrhage (ICH) on follow-up imaging and an increase of ≥4 points on the NIHSS from baseline or the lowest value within 7 days, or mortality.
Intraarterial thrombectomy
Angiographic images (Artis Q biplane; Siemens Medical Systems, Erlangen, Germany) were obtained using standard techniques. The right side groin was prepped and draped in a sterile manner. The femoral artery was catheterized with an 8-Fr sheath and 8-Fr balloon guide catheter, and a 5-Fr headhunter catheter was used to identify the target artery. If the vasculature was tortuous, a distal access catheter was also inserted through the guiding catheter. Multiple runs (providing multiple views) were performed to identify the occlusion site. The diseased segment was catheterized selectively and intensively using an Excelsior XT-27 microcatheter (Boston Scientific, Natick, MA, USA) and Synchro micro-guidewire (Boston Scientific). Mechanical thrombectomy was performed using a Solitaire FR (ev3; Covidien, Irvine, CA, USA) or Trevo stent retriever (Stryker, Kalamazoo, MI, USA). The stent usually remained in situ for approximately 5 minutes before being retrieved through aspiration via a balloon guiding or distal access catheter. If the occlusion site did not open, this procedure was repeated. However, in some patients, re-occlusion occurred after recanalization. In those patients who experienced two or three re-occlusions after recanalization, a stent retriever was deployed at the occluded site. Following the IA-Tx procedure, all patients were admitted to the neurosurgical intensive care unit for several days. Successful recanalization was graded according to the TICI scale, and a TICI grade higher than 2b was defined as successful recanalization.
Surgical indications and postoperative management
The DC indications were as follows : significant unilateral brain swelling evident on CT scans with associated clinical deterioration; a decrease in GCS scores (to ≤8) and/or dilated pupils unresponsive to light; a midline shift of >6 mm; and/or obliteration of cisternal structures on CT [ 47]. Patients with primary fatal brainstem failure (indicated by an initial GCS score of 3 that remained unchanged thereafter and/or bilaterally fixed and dilated pupils) did not undergo decompression. Neurologic status was evaluated by GCS score because NIHSS did not accurately reflect the patient’s mental status, and most previous papers that evaluated the patient’s neurologic condition regarding DC surgery used GCS score. After DC surgery, conventional medical management, including hyperosmotic agents, hyperventilation, and extraventricular drainage (EVD), was initiated if the intracranial pressure exceeded 20 mmHg.
Surgical procedures
The patient was placed in a supine position, and a ventricular puncture was made at the Kocher point opposite the lesion. An EVD catheter (Yushin Medical, Seoul, Korea) was connected to a continuous cerebral perfusion pressure (CPP) monitor (Spiegelberg, Hamburg, Germany) through a transducer (Druckmesset; Smiths Industries, Kirchseeon, Germany), for precise and continuous measurement of the mean ventricular pressure. Skin flaps were positioned behind the parietal eminence and extended downward to the zygoma; they were continued subperiosteally to the level of the supraorbital ridges. After stabilizing the ventricular ICP, a saw was used to connect the keyholes and thus create the bone flap. The frontal median segment of the bone (approximately 3-4 cm in width along the sagittal sinus) was preserved to avoid damaging the sinus and to provide a structure for subsequent cranioplasty. Additional bone was removed from the temporal region to the floor of the middle fossa. The dura was then opened through a large cruciate or curved Z-shaped incision in the areas of the frontal, temporal, and parietal lobes. When the dura was opened, the underlying brain typically herniated outward. Cortical brain resection was not performed. In all patients, a synthetic dura (Lyoplant; Braun Melsungen AG, Melsungen, Germany) was placed underneath the incised dura and secured with several sutures to allow the brain to herniate outward in a more controlled manner and to prevent cortical adhesion. In addition, thin Surgicel pieces were placed between the skin flap and dura to prevent cortical adhesion and allow easy dissection during the subsequent cranioplasty. The temporalis muscle and skin flap were then re-approximated with sutures ( Fig. 2) [ 47].
Data collection
The NIHSS and GCS were used to evaluate neurological status in the emergency room. Additionally, the GCS was used for assessing neurological status in the intensive care unit before and after the DC surgery. A neuroradiologist measured the midline shift on CT scans taken within 3 hours before the DC procedure. The midline shift refers to the distance from the septum pellucidum to the midline between the anterior and posterior edges of the falx at the point where the falx attaches to the inside of the calvarium. The initial ICP was recorded after the ventricle had been punctured, reflecting the highest sustained ventricular pressure. The ventricular pressure was monitored continuously during surgery and the postoperative period (2-7 days). Clinical outcomes were evaluated based on mRS scores. A favorable clinical outcome was defined as an mRS score of 0-2 at 90 days, while an unfavorable outcome was defined as an mRS score of 3-5 (6, deceased) at the same time point. To avoid observer bias, all mRS scores were calculated by two separate blinded investigators based on notes written by an independent, unblinded nurse who visited each patient and their relatives. In case of disagreement, the final mRS score was decided by consensus.
Statistical analysis
All data is presented as means±standard deviations and/or medians. The Wilcoxon signed-rank test was used to analyze the NIHSS, GCS, and mRS. The unpaired t-test and Fisher’s exact test were used to compare groups. The GCS score was analyzed using Fisher’s Exact test for every GCS 1 value. The lowest GCS value with a significant p-value was analyzed. All statistical analyses were carried out using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA). The significance level was set at p≤0.05.
RESULTS
The mean patient age was 56.4±14.4 years (range, 20-86 years; median, 56 years), and 39 of the 67 patients (58.2%) were male ( Table 1).
Neurological outcomes according to neurological status immediately before DC surgery
The overall mortality rate was 31.3% (21/67), the unfavorable outcome rate was 44.8% (30/67), and the favorable outcome rate was 23.9% (24/67). We compared the clinical outcomes based on the preoperative neurological status, specifically, a GCS score of either above or below 7. Regarding favorable neurological outcomes based on neurological status just before DC, a GCS score of 7 was the lowest value that held statistical significance (Fishers’ Exact test : GCS ≥9, p=0.224; GCS ≥8, p=0.047; GCS ≥7, p=0.013; GCS ≥6, p=0.095) ( Table 2). Preoperative neurological status did not correlate with mortality (GCS ≥9, p=0.687; GCS ≥8, p=0.468; GCS ≥7, p=0.063; GCS ≥6, p=0.133).
Neurological outcomes according to surgical time
The surgical time window (p=0.804) did not correlate with favorable neurological outcomes. According to the chi-squared test, a favorable neurological outcome was not associated with any time windows (<12 hours, p=0.286; 18 hours, p=0.337; 24 hours, p=0.079; 36 hours, p=0.389; 48 hours, p=0.283).
Neurological outcomes according to P/D mismatch
The favorable outcome rate was 36.4% (12/33) in P/D-mismatched patients and 8.8% (3/34) in P/D-matched patients (p=0.007). The mortality rate was 3% (1/33) in P/D-mismatched patients and 58.8% (20/34) in P/D-matched patients (p=0.000). The recanalization and significant hemorrhagic rates were 78.8% (26/33) and 3% (1/34), respectively, in P/D-mismatched patients and 20.6% (7/34) and 44.1% (15/34), respectively, in P/D-matched patients (both p<0.001).
Other correlations with patient characteristics
Favorable neurological outcomes were correlated with the initial ICP (p<0.001), recanalization of the occluded vessel (p<0.001), and midline shift (p=0.021). Recanalization of the LVO after IA-Tx was positively correlated with a favorable neurological outcome. Among the patients for whom recanalization was successful after IA-Tx and IV-tPA, 39.4% (13/33) had favorable neurological outcomes, compared to 5.9% (2/34, p=0.001) of the non-recanalized patients. The clinically significant hemorrhagic rate was 3% (1/34) in P/D-mismatched patients and 44.1% (15/34) in P/D-matched patients (p<0.001). The neurological outcome also correlated significantly with P/D-mismatch on MRI after IV-tPA (but before IA-Tx). The favorable neurological outcome rate was 36.4% (12/33) in P/D-mismatched patients but only 8.8% (2/34) in P/D-matched patients (p=0.007).
DISCUSSION
The principle of ischemic stroke treatment is reperfusion of the occluded vessel and the preservation of vulnerable tissues [ 1, 2, 4, 9, 13, 16, 32, 34, 35]. Over the past few decades, a variety of methods have been developed to treat ischemic stroke. Initially, IV-tPA administration within 3 hours of symptom onset in patients without contraindications was the treatment of choice for ischemic stroke. Subsequently, the critical therapeutic window was extended to up to 4.5 hours. Some studies report the combination of IV and IA therapy [ 5, 13, 22, 29, 31, 32, 36, 39, 45, 48]. However, occlusions of large, proximal intracranial arteries usually do not recanalize after IV-tPA administration. Some early studies reported that early reperfusion occurred after IV-tPA administration in only 13-50% of patients with occlusions in the internal cerebral artery terminus or proximal segment of the middle cerebral artery [ 14, 39, 43]. After the MR CLEAN study was published, the recanalization rate and neurological outcomes after IA-Tx in LVO patients significantly improved compared to those after IV-tPA alone. Recently, with the rapid development of endovascular devices and increased clinical experience, the recanalization rate has increased to over 95% [ 5, 29, 31, 39, 48]. Pathological brain swelling varies among individuals, and in some cases, successful recanalization can still occur after brain swelling is processed [ 7, 8, 17, 23, 24, 28, 37, 46, 49]. Early DC surgery (within a defined time window) may be beneficial, but it could be unnecessary and overly aggressive in some patients ( Fig. 3). The indications for DC in patients with major infarctions must be refined because the therapeutic strategy has changed. The pathological processes of the ischemic injured tissue are influenced by various treatment strategies [ 10, 12, 18, 19, 21, 26, 27, 39, 42- 44]. However, in some instances, despite successful recanalization, reperfusion injury has been reported [ 24, 28]. Several treatment options have both advantages and disadvantages when used to control massive brain swelling caused by traumatic brain injury, LHI, ICH, aneurysmal subarachnoid hemorrhage (SAH), and other events. As the brain is encased by a rigid bony structure, brain edema of any etiology increases the ICP, leading to adverse outcomes, including death. Therapies that normalize ICP improve the CPP, CBF, and other brain hemodynamics, allowing for better outcomes in critically ill patients. In many clinical and animal studies, DC was used to remove a large area of skull bone, thus transforming the intracranial space (a “closed box” with a finite volume) into an “open box” to improve brain compliance in terms of pressure and volume. DC is an advanced treatment option for ICP control that allows the CPP and CBF to be maintained at levels that prevent ischemic injury. Also, the edematous brain herniates through the craniectomy opening rather than the tentorial incisura, thus avoiding brainstem compression [ 8, 11, 17, 31, 37, 43, 47, 49]. Many studies used DC to treat patients with LHI caused by LVO; early DC (within 36-48 hours of symptom onset) was more effective than medical treatment for improving survival and neurological outcomes [ 8, 10, 12, 18- 20, 27, 36, 39, 49, 50]. Several studies have reported that brain swelling is greatest within 2-3 days after an ischemic insult. A space-occupying mass typically develops over the following 5 days [ 7, 23, 37, 49]. Several papers have described clinical, radiographic, and laboratory characteristics that predict massive brain edema caused by hemispheric infarction. In our study, 15 patients underwent DC surgery more than 48 hours after initial treatment; two exhibited favorable outcomes and four died. In our study, among patients treated with DC after IA-Tx and IV-tPA, 22.4% (15/67) had favorable outcomes, and the mortality rate was 31.3% (21/67) at the 3-month neurological outcome assessment. Those with GCS scores ≥7 just before DC, the lowest neurological status, can expect a favorable outcome ( p=0.013). IA-Tx is usually performed in LVO patients according to time window guidelines or imaging studies. Patients with good collateral circulation and successful recanalization have more favorable neurological outcomes; in those for whom IA-Tx fails and the collateral circulation is low, the outcomes are poor. Our results accorded with those findings ( Table 1). Favorable outcomes correlated significantly with successful recanalization after IA-Tx ( p=0.001) and P/D-mismatch evident on MRI performed immediately prior to IA-Tx ( p=0.007) [ 8, 19, 31, 36, 48]. In our study, the surgical time window ( p=0.469) did not correlate with a favorable neurological outcome [ 40]. While previous studies reported that the DC time window for LHI patients was important to the neurological outcome. In our study, 15 patients underwent DC later than 48 hours after initial treatment. These late DC patients exhibited recanalization ( p=0.302), P/D-mismatch ( p=0.208), favorable outcome ( p=0.283), and mortality rates ( p=0.458) similar to those of the early (within 48 hours) DC group. Recently, several studies reported that DC is effective on patient survival after IA-Tx, but these papers had not weighted much on neurologic status, as in our analysis [ 8, 38, 46]. Authors propose that both neurological status and the surgical time window should be considered when contemplating DC for patients with LHI after IA-Tx, with or without IV-tPA. We used the GCS rather than NIHSS to evaluate neurological status because the GCS better reflects changes in mental status and is the recognized standard for managing neurosurgical patients (including those with traumatic brain injuries). In clinical practice, some patients with major infarctions caused by LVO have good neurological outcomes without DC. Thus, we suggest that very early DC may sometimes be unnecessary. We found that a GCS score ≥7 after IA-Tx and IV-tPA was the lowest score associated with a favorable outcome after DC. Recent reports have shown that successful recanalization reduces the need for DC [ 3, 38]. We found that the neurological outcomes of DC patients who were successfully recanalized after IA-Tx (with or without IV-tPA) were more favorable than those of non-recanalized patients. However, although the recanalization rate increased after IA-Tx, the reperfusion injury rate increased [ 28, 33]. Minimizing reperfusion injury after IA-Tx is important, and P/D-mismatching is significant for outcome and reperfusion injury. The initial ICP correlated significantly with both the initial neurological status ( p=0.033) and final neurological outcome ( p=0.000). This study had several limitations. First, there was no control group; we could not analyze the survival rate for patients with favorable neurological outcomes without DC according to the reperfusion injury rate. Also, we lacked data allowing comparison of pathological status before and after recanalization because the surgical time windows varied after additional IA-Tx.
CONCLUSION
To minimize the need for unnecessary DC after a cerebral infarction, it’s crucial to take into account both the neurological status and the surgical time window. Successful recanalization also positively impacts patient outcomes. We propose that a GCS score of 7 is the minimum score that justifies the use of DC to treat LHI following IA-Tx. Recanalization of the obstructed vessel and P/D-mismatch are significant factors in improving the long-term neurological outcomes of patients treated with IA-Tx, with or without IV-tPA.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science (NRF-2022R1F1A1076411) and The Catholic University of Korea, Eunpyeong St. Mary’s Hospital, Research Institute of Medical Science in program year 2021, 2022.
Authors appreciate the help of neurosurgical nurse specialist Hye-Seon Jeong R.N. for collecting the data by and the grammatical review of our manuscript by Ann C. Rice, PhD of J. Sargeant Reynolds Community College, Richmond, VA, USA.
Fig. 1.
Flow diagram of the treatment protocol. CT : computed tomography, IV-tPA : intravenous tissue plasminogen activator, MRI : magnetic resonance imaging. 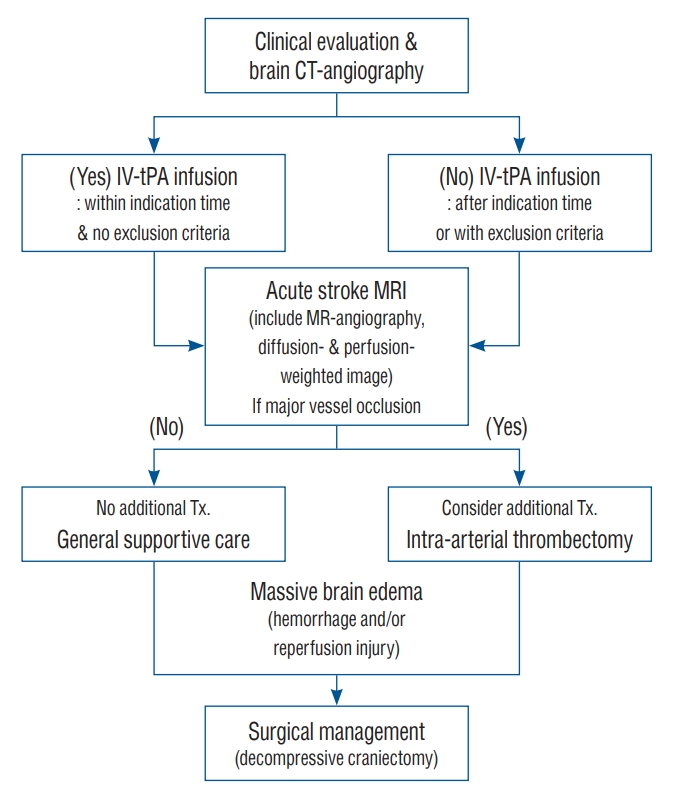
Fig. 2.
A : A preoperative brain computed tomography (CT) image reveals massive brain edema, midline shift, and reperfusion injury of the right cerebral hemisphere after intraarterial thrombectomy. B : Brain CT images taken after right side decompressive hemicraniectomy and insertion of extraventricular drainage catheter. C : A three dimensional-reconstructed CT image after decompressive craniectomy. The image shows a large skull bone defect on the right side, and the burr hole at the left Kocher's point for extra-ventricular drainage. 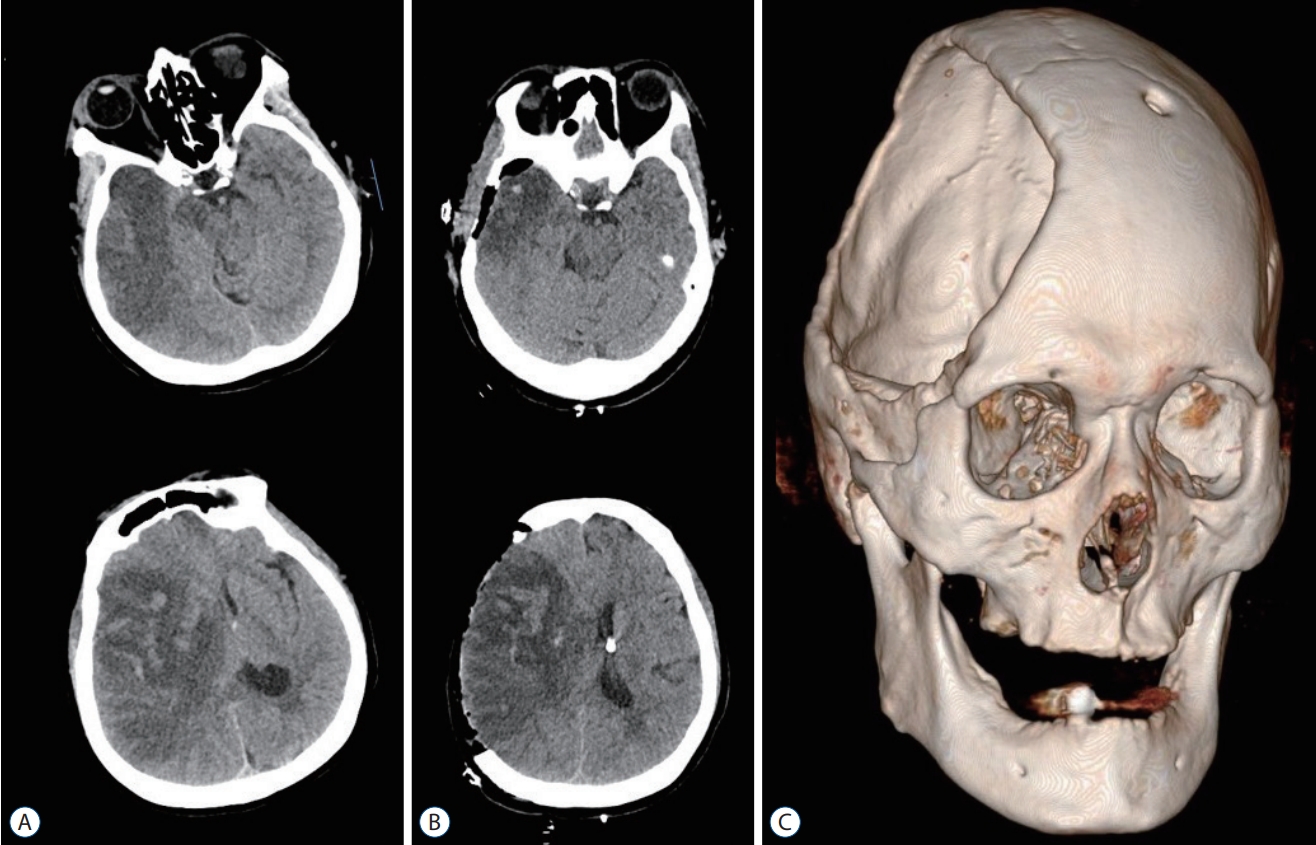
Fig. 3.
Patient who doesn’t need not decompressive craniectomy (DC) after major infarction. brain computed tomography (CT) image (upper row) initial brain CT at ictus (29/March), six follow-up brain CT without DC (9/April) (lower row). 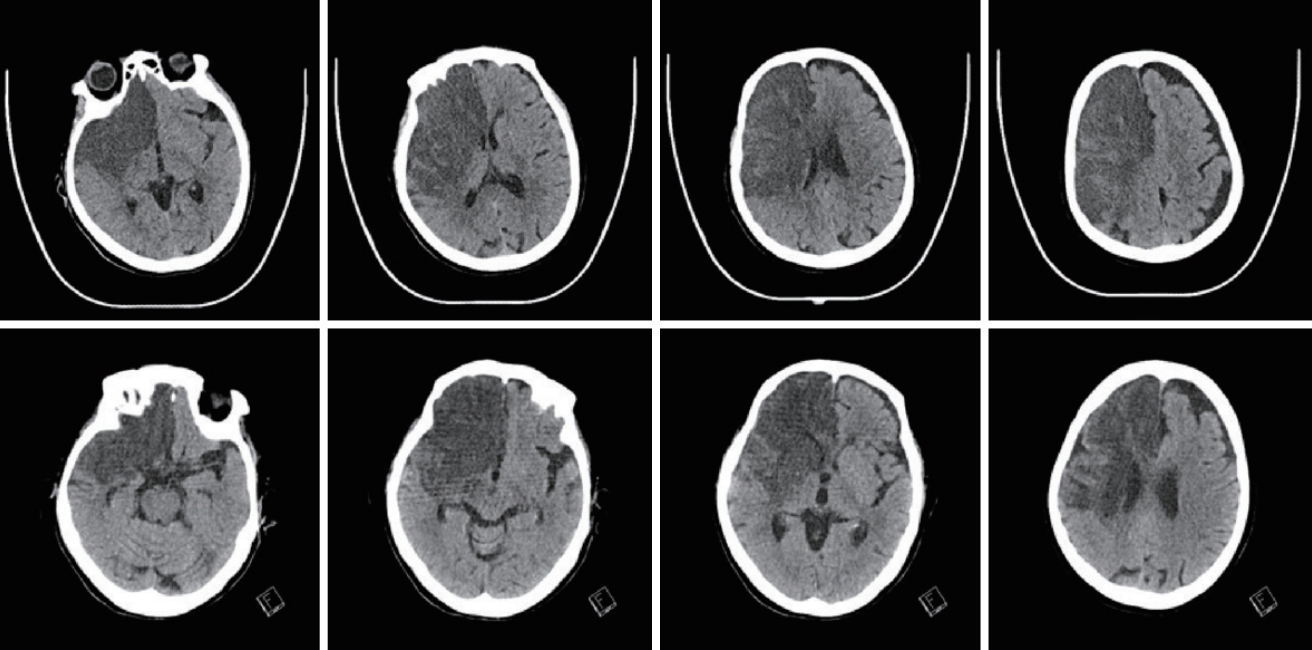
Table 1.
Patient demography and neurologic outcomes after IA-Tx and IV-rtPA
|
Total |
Recanalized |
Non-recanalized |
p-value |
|
Patients No. |
67 |
33 |
34 |
|
|
Age (years) |
56.4±14.4 (56, 20-86) |
58.1±16.0 (60, 20-79) |
54.7±12.6 (54, 38-86) |
0.149 |
|
Male |
39 (58.2) |
21 (63.6) |
18 (52.9) |
0.261 |
|
Initial GCS |
6.6±1.8 (7) |
6.7±2.1 (8) |
6.6±1.4 (6) |
0.030*
|
|
IV-rtPA time (minutes) |
119.1±41.3 (120) |
117.6±40.6 (120) |
120.6±42.6 (120) |
0.711 |
|
IA-Tx time (hours) |
4.6±0.9 (4.5) |
4.6±0.8 (4.5) |
4.5±1.0 (5.0) |
0.012 |
|
OP time interval (hours) |
37.8±38.4 (29) |
35.5±21.9 (36) |
40.0±49.7 (26) |
0.263 |
|
Mismatching, matching |
33 (49.3) |
26 (78.8) |
7 (20.6) |
<0.001 |
|
Initial ICP (mmHg) |
30.5±12.9 (27) |
26.3±11.6 (23) |
34.5±13.0 (32) |
0.395 |
|
Midline shift |
13.7±5.3 (13.3) |
13.3±4.0 (13.0) |
14.0±6.4 (13.3) |
0.043*
|
|
mRS |
|
|
|
|
|
0 |
6 (9.0) |
5 (15.2) |
1 (2.9) |
|
|
1 |
4 (6.0) |
4 (12.1) |
- |
|
|
2 |
6 (9.0) |
5 (15.2) |
1 (2.9) |
|
|
Favorable |
14 (23.9) |
13 (39.4) |
2 (5.9) |
<0.001*
|
|
3 |
12 (17.9) |
8 (24.2) |
4 (11.8) |
|
|
4 |
5 (7.5) |
3 (9.1) |
2 (5.9) |
|
|
5 |
13 (19.4) |
5 (15.2) |
8 (23.5) |
|
|
Mortality |
21 (31.3) |
3 (9.1) |
18 (52.9) |
0.000*
|
|
Sig-Hx |
16 (23.9) |
4 (12.1) |
12 (35.3) |
0.025 |
Table 2.
Favorable outcome according to the preoperative GCS
|
Favorable |
Unfavorable |
p-value |
|
GCS ≥9 |
14 |
52 |
0.224 |
|
GCS ≥8 |
13 |
52 |
0.047*
|
|
GCS ≥7 |
11 |
33 |
0.013*
|
|
GCS ≥6 |
4 |
26 |
0.095 |
References
1. Adams HP Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al : Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke 34 : 1056-1083, 2003   2. Adams HP Jr, Brott TG, Crowell RM, Furlan AJ, Gomez CR, Grotta J, et al : Guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 25 : 1901-1914, 1994   3. Akins PT, Axelrod YV, Arshad ST, Guppy KH : Initial conservative management of severe hemispheric stroke reduces decompressive craniectomy rates. Neurocrit Care 25 : 3-9, 2016    4. Astrup J, Siesjö BK, Symon L : Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 12 : 723-725, 1981   5. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372 : 11-20, 2015  6. Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D : Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med 24 : 620-623, 1998    7. Bounds JV, Wiebers DO, Whisnant JP, Okazaki H : Mechanisms and timing of deaths from cerebral infarction. Stroke 12 : 474-477, 1981   8. Daou B, Kent AP, Montano M, Chalouhi N, Starke RM, Tjoumakaris S, et al : Decompressive hemicraniectomy: predictors of functional outcome in patients with ischemic stroke. J Neurosurg 124 : 1773-1779, 2016   9. Davis S, Donnan GA : Time is penumbra: imaging, selection and outcome. The Johann jacob wepfer award 2014. Cerebrovasc Dis 38 : 59-72, 2014    10. Delashaw JB, Broaddus WC, Kassell NF, Haley EC, Pendleton GA, Vollmer DG, et al : Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke 21 : 874-881, 1990   11. Eun J, Huh J, Yang SY, Huh HY, Ahn JK, Cho KW, et al : Determining the lower limit of cerebral perfusion pressure in patients undergoing decompressive craniectomy following traumatic brain injury. World Neurosurg 111 : e32-e39, 2018   12. Frank JI, Schumm LP, Wroblewski K, Chyatte D, Rosengart AJ, Kordeck C, et al : Hemicraniectomy and durotomy upon deterioration from infarction-related swelling trial: randomized pilot clinical trial. Stroke 45 : 781-787, 2014    13. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al : Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 282 : 2003-2011, 1999   14. Gorelick PB, Wong KS, Bae HJ, Pandey DK : Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 39 : 2396-2399, 2008   15. Göttsche J, Flottmann F, Jank L, Thomalla G, Rimmele DL, Czorlich P, et al : Decompressive craniectomy in malignant MCA infarction in times of mechanical thrombectomy. Acta Neurochir (Wien) 162 : 3147-3152, 2020    16. Hacke W, Kaste M, Skyhoj Olsen T, Orgogozo JM, Bogousslavsky J : European Stroke Initiative (EUSI) recommendations for stroke management. The European Stroke Initiative Writing Committee. Eur J Neurol 7 : 607-623, 2000   17. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R : ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 53 : 309-315, 1996   19. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, et al : Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 8 : 326-333, 2009   20. Hofmeijer J, van der Worp HB, Kappelle LJ : Treatment of space-occupying cerebral infarction. Crit Care Med 31 : 617-625, 2003   21. Holtkamp M, Buchheim K, Unterberg A, Hoffmann O, Schielke E, Weber JR, et al : Hemicraniectomy in elderly patients with space occupying media infarction: improved survival but poor functional outcome. J Neurol Neurosurg Psychiatry 70 : 226-228, 2001    23. Hossmann KA : Ischemia-mediated neuronal injury. Resuscitation 26 : 225-235, 1993   24. Hussein HM, Georgiadis AL, Vazquez G, Miley JT, Memon MZ, Mohammad YM, et al : Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol 31 : 454-458, 2010    25. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al : Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375 : 1119-1130, 2016   26. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al : Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 38 : 2518-2525, 2007   27. Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW, et al : Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med 370 : 1091-1100, 2014   28. Larrue V, von Kummer R, Müller A, Bluhmki E : Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 32 : 438-441, 2001   29. Lewandowski CA, Frankel M, Tomsick TA, Broderick J, Frey J, Clark W, et al : Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: emergency management of stroke (EMS) bridging trial. Stroke 30 : 2598-2605, 1999   30. Lin J, Frontera JA : Decompressive hemicraniectomy for large hemispheric strokes. Stroke 52 : 1500-1510, 2021   31. Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallée PC, et al : Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol 8 : 802-809, 2009   32. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group : Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333 : 1581-1587, 1995   33. Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J : Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 49 : 93-102, 2007    34. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al : 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46 : 3020-3035, 2015  35. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al : 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49 : e46-e110, 2018  36. Rha JH, Saver JL : The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 38 : 967-973, 2007   37. Ropper AH, Shafran B : Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch Neurol 41 : 26-29, 1984   38. Rumalla K, Ottenhausen M, Kan P, Burkhardt JK : Recent nationwide impact of mechanical thrombectomy on decompressive hemicraniectomy for acute ischemic stroke. Stroke 50 : 2133-2139, 2019   39. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al : Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke 29 : 1888-1893, 1998   40. Slezins J, Keris V, Bricis R, Millers A, Valeinis E, Stukens J, et al : Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant middle cerebral artery stroke. Medicina (Kaunas) 48 : 521-524, 2012   41. Tracol C, Vannier S, Hurel C, Tuffier S, Eugene F, Le Reste PJ : Predictors of malignant middle cerebral artery infarction after mechanical thrombectomy. Rev Neurol (Paris) 176 : 619-625, 2020   42. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al : Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6 : 215-222, 2007   43. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al : Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 38 : 2506-2517, 2007   44. Walz B, Zimmermann C, Böttger S, Haberl RL : Prognosis of patients after hemicraniectomy in malignant middle cerebral artery infarction. J Neurol 249 : 1183-1190, 2002    45. Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al : Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 379 : 2364-2372, 2012    46. Wu S, Yuan R, Wang Y, Wei C, Zhang S, Yang X, et al : Early prediction of malignant brain edema after ischemic stroke. Stroke 49 : 2918-2927, 2018   47. Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK : Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg 91 : 953-959, 1999   48. Yoo DS, Won YD, Huh PW, Shin HE, Kim KT, Kang SG, et al : Therapeutic results of intra-arterial thrombolysis after full-dose intravenous tissue plasminogen activator administration. AJNR Am J Neuroradiol 31 : 1536-1540, 2010    49. Yuan R, Wu S, Cheng Y, Ye K, Hao Z, Zhang S, et al : Association between preoperative midline shift growing rate and outcomes of decompressive craniectomy in patients with malignant middle cerebral artery infarction. Curr Neurovasc Res 17 : 131-139, 2020   50. Zhao J, Su YY, Zhang Y, Zhang YZ, Zhao R, Wang L, et al : Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 17 : 161-171, 2012   
|
|



















