Circi, Atici, Baris, Senel, Leblebici, Tekin, and Ozturkmen: Is Tranexamic Acid an Effective Prevention in the Formation of Epidural Fibrosis? Histological Evaluation in the Rats
Abstract
Objective
The present study aimed to determine the topical and systemic efficacy of tranexamic acid (TXA) on epidural fibrosis in a rat laminectomy model.
Methods
Thirty-two 12-month-old adult Sprague-Dawley rats were used in this study. Each rat underwent bilateral laminectomy at the L1 and L2 vertebral levels. Rats were divided into four groups : in group I (control group, n=8), a laminectomy was performed and saline solution was applied into the surgical space. In group II (topical group, n=8), laminectomy was performed and 30 mg/kg TXA was applied to the surgical site before skin closure. In group III (systemic group, n=8), 30 mg/kg TXA was administered intravenously via the tail vein in the same session as the surgical procedure. In group IV (topical and systemic group, n=8), TXA was administered 30 mg/kg both topical and intravenous. The rats were sacrificed at 4 weeks postoperatively. Masson’s trichrome and hematoxylin and eosin were used to assess acute inflammatory cells, chronic inflammatory cells, vascular proliferation, and epidural fibrosis.
Results
Epidural fibrosis, acute inflammation, chronic inflammation, and sum histologic score value were significantly lower in the systemic TXA group, systemic and topical TXA groups than in the control group (p<0.05). In addion, the sum histologic score was significantly lower in the topical TXA group than in the control group (p<0.05).
Conclusion
In this study, epidural fibrosis formation was prevented more by systemic application, but the topical application was found to be effective when compared to the control group. As a result, we recommend the systemic and topical use of TXA to prevent epidural fibrosis during spinal surgery.
Key Words: Laminectomy · Tranexamic acid · Rats · Epidural fibrosis.
INTRODUCTION
Failed back surgery syndrome is well-defined as the increase or persistence of complaints such as leg pain and low back after spinal surgery. Epidural fibrosis has been shown to be the reason of symptoms in 6-24% of failed back surgery patients [ 12, 22, 23]. Epidural fibrosis is the formation of fibrous tissue in the epidural space. Many stages are involved in the formation of epidural fibrosis. Because of tissue damage due to surgery, spinal epidural hematoma may accumulate when it is absorbed and gradually replaced by granulation tissues. Eventually, the granulation tissue matures and is replaced by fibrous tissue in the epidural space. Developing scar tissue can retract or compress the nerve roots and/or dural sac [ 1, 4, 7, 20]. Previous studies show that the amount of postoperative epidural fibrosis is associated with the worsening of patient complaints [ 25, 34]. There are studies on many substances to prevent epidural fibrosis, and there is no consensus reached yet. However, the researchers have emphasized the importance of preventing epidural fibrosis in spinal surgery [ 3, 6, 9, 12, 20, 30, 33, 34]. Tranexamic acid (TXA), first presented to medical practice in the 1960s, is an antifibrinolytic agent that acts by binding to plasminogen and blocking the contact of plasminogen with formed fibrin clot and reduces active bleeding [ 10]. Perioperative and postoperative blood loss is common in major orthopedic surgery, especially in hip and knee arthroplasty and spine surgery. TXA was widely used in spine surgeries to decrease bleeding, mortality, and morbidities related to transfusion requirements. TXA can be administered orally, intravenously, and topically, it was seen that the most common method of administration intravenously in the literature. There is no consensus on the method of administration and dosage [ 3, 10, 16, 30, 33]. Although there are many studies on TXA in the literature, to our knowledge no study to date has examined experimentally the effect of both topical and systemic TXA application in the formation of epidural fibrosis. It can be considered that TXA provided by hemostasis, reduces the grade of epidural fibrosis following laminectomy. The present study aimed to determine the effect of topical and systemic TXA in the formation of epidural fibrosis in an experimental rat laminectomy model.
MATERIALS AND METHODS
International and national protocols were followed in the treatment of the animals. The study was approved all procedures by the Institutional Animal Care and Use Committee (IACUC) of Bezmialem University under license number 2022/77. In our study, 32 adult female Sprague-Dawley rats aged 12 months with a mean weight of 300±49 g were used. The rats were fed a standart diet and given unlimited access to water in confined laboratory settings. The temperature and humidity levels in the room were kept at 20°C to 24°C and 50% to 60%, respectively. A 12-hour light cycle was chosen.
Each rat has a unique identification mark. Ketamine hydrochloride (35 mg/kg; Ketalar; Pfizer, Istanbul, Turkey) and xylazine (10 mg/kg; Rompun; Bayer, Istanbul, Turkey) were injected intramuscularly to induce anesthesia. The rats were positioned in the prone and surgical site were shaved. Using povidone-iodine, the surgical area was made sterile (Batticon; Adeka Pharmaceuticals, Istanbul, Turkey). A median skin incision was performed from the T12 to the L3 vertebrae. Bilateral laminectomy was performed at the L1 and L2 vertebral levels using a microscope with an ×2.5 magnification (World Precision Instruments, Sarasota, FL, USA) ( Fig. 1). The dura mater and nerve roots were visible. Four groups were created at random from the rats. A laminectomy was done and saline solution was injected into the intervertebral space in group I (control group, n=8). Laminectomy was carried out in group II (topical medication group, n=8) before the skin was closed, and 30 mg/TXA (Herajit; Vem, Ankara, Turkey) was applied to the surgical site. Group III (systemic drug group, n=8) received 30 mg/kg of TXA intravenously through the tail vein during the same session as the surgery. TXA was given topically and intravenously in group IV (topical and systemic medication group, n=8) at a dose of 30 mg/kg.
Tha facia and skin incision were closed with a 3.0 sutures after meticulous hemostasis had been accomplished. For preoperative and postoperative antibiotic prophylaxis, an intraperitoneal injection of 10 mg/kg of enrofloxacin (Baytril-K; Bayer HealthCare AG, Leverkusen, Germany) was given. For postoperative analgesia, fentanyl 0.02 mg/kg (Fentanyl; Janssen-Cilag Pty Ltd, North Ryde, Australia) was supplied intraperitoneally. Rats’ motor functions were normal, and no signs of systemic pathology or wound infection were found.
In the fourth week, rats were sacrificed using 100 mg/kg of ketamine hydrochloride (Ketalar; Pfizer). The lumbar spine was completely removed from the rats after thorough dissection.
To enable sectioning, tissue samples were put in a 10% neutral buffered formalin solution and subsequently decalcified in 10% formic acid for 7 days. Axial sections were obtained from the laminectomy area after decalcification. The fixed tissue underwent processing, paraffin embedding, and 3 µm sectioning. Double-blind histopathological analysis including the following parameters was performed by the pathologist.
Tissue sections were stained with Hematoxylin and Eosin to assess acute, chronic inflammation and vascular proliferation. Histologic analysis was presented on a 4-point grading system consistent with the following scale : 0, indicates the laminectomy defect was no parameter; 1, indicates local involvement of the laminectomy defect; 2, indicates mild or moderate involvement of the laminectomy defect; and 3, indicates a parameter covering the whole of the laminectomy side [ 3]. To evaluate epidural fibrosis, Masson’s trichrome staining was used. We employed the evaluation method outlined by He et al. [ 9] in the related publication to evaluate the severity of epidural fibrosis and the condition of the dura mater. Grade 0, no scar tissue extending to the dura mater; grade 1, a thin fibrous band is observed between the scar tissue and the dura mater; grade 2, fibrous tissue is observed covering less than two-thirds of the laminectomy defect; and grade 3, fibrous tissue is covered the entire laminectomy defect and spread to the nerve roots [ 9].
Statistical analysis
In the descriptive statistics of the data, mean, standard deviation, median, minimum, maximum, frequency, and ratio values were used. Using the Kolmogorov-Smirnov test, the distribution of the variables was evaluated. Kruskal-wallis, Mann-Whitney U test was used in the analysis of quantitative independent data. The results were accepted as being statistically significant at p<0.05. SPSS ver. 28.0 program (SPSS Inc., Chicago, IL, USA) was used in the analysis.
RESULTS
The general condition of the rats was good; no complications were encountered such as infection, dural tear or paralysis after laminectomy. The mean histological grade and standard deviation, the median value, sum of the histological score of the groups, and statistical analyses are presented in Tables 1 and 2. Sum histologic score represents the sum of the mean values of each histological evaluation parameter. Histologic grades of the study groups were analyzed separately. Acute inflammation, chronic inflammation, epidural fibrosis, and sum histologic score were significantly lower in the systemic TXA group and systemic with topical TXA group compared to the control group (p<0.05). Vascular Proliferation value did not differ significantly (p>0.05) between the groups. Although epidural fibrosis, and sum histologic score values were lower in the systemic and topical TXA group than in the systemic TXA group, no statistically significant difference was found between the results (p>0.05). Acute and chronic inflammation, and epidural fibrosis did not differ significantly between the control and the topical TXA group (p>0.05).
In Fig. 2A and B shows grade 3 vascular proliferation sites at the interface between the dura and scar tissue. In Fig. 2A and B also indicates dura mater was free of scar tissue in the systemic and topical TXA group. In Fig. 2C and D show grade 3 epidural fibrosis covered the whole laminectomy defect with a wide adherence to dura mater from the control group. It was determined that the sum histologic score was lower in the systemic with topical TXA and the systemic TXA group copared to control group ( p<0.05). Also, the sum histologic score was significantly ( p<0.05) lower in the group using topical TXA than in the control group. Comparisons of the mean histologic sum score among groups are summarized in Fig. 3.
DISCUSSION
Persistent low back pain encountered after lumbar spine surgeries causes many economic, psychological, and social problems for both the patient and the doctor. One of the most important causes of pain is epidural fibrosis. The incidence of epidural fibrosis after laminectomy ranges from 10% to 75% [ 30]. Many factors affect the formation of epidural fibrosis. It is well described that inadequate bleeding control and the presence of blood products in the epidural space during surgery facilitate the formation of epidural fibrosis while it is a appearance of the normal progress of wound healing. Macrophages, which migrate from the bone marrow to the circulation as monocytes and then settle in the tissues, are the most important cells in chronic inflammation. Macrophages are responsible for the release of mediators that cause tissue damage and the release of agents that cause fibrosis. Lymphocytes responsible for chronic inflammation secrete various cytokines and provide monocyte and macrophage activation. Unlike acute inflammation, tissue destruction and fibrosis are prominent in chronic inflammation. Fibrosis caused by fibroblast proliferation and excess extracellular matrix collection is an important cause of organ dysfunction [ 18]. Many experimental studies have been done to prevent epidural fibrosis and very limited clinical and practical use on a routine basis [ 3, 6, 9, 12, 20, 30, 33, 34]. In our study, we evaluated the effects of TXA on the formation of epidural fibrosis, with the thought that controlling bleeding in the epidural space will prevent fibrosis. It has been predicted that use of systemic TXA decrease in bleeding in the epidural space, may cause a decrease in the fibrous tissue so ultimately reduces epidural fibrosis. It was thought that the desired clinical benefit with TXA could be achieved by reducing the fibrous tissue that causes compression on the spinal cord after laminectomy. In our study, we planned to determine the effect of topical use as well as the widely used systemic route of administration. In our study, we found that both systemic and topical TXA applications are more effective in preventing epidural fibrosis when used together. Although no statistical difference was detected between the topical TXA application and the control group, the amount of epidural fibrosis was found to be lower in the topical TXA group. Another interesting result of the study was that the sum histologic score was found to be significantly lower in the topical TXA group compared to the control group. Therefore, we can say that both topical and systemic TXA use is effective in preventing epidural fibrosis. According to a comprehensive review, systemic TXA application is effective in reducing surgical bleeding in spinal surgery and topical TXA in surgery suggests similar hemostatic efficacy and possibly improved safety as compared with systemic TXA [ 32]. Li et al. [ 15] revealed both intravenous and topical use of TXA effectively reduced surgical blood loss compared to intravenous or topical use alone in patients planned for lumbar fusion surgery, without increasing the incidence of thromboembolic events. Xu et al. [ 33] revealed that collagen sponge and topical TXA have both proven to be effective in control bleeding in posterior fusion surgery in the clinical study. Researchers also reported that TXA has showed better efficacy. A combination of TXA and acute normovolemic hemodilution might be an successful plan for reducing blood loss in patients undergoing lumbar spinal fusion surgery [ 16]. We organized this study by predicting that TXA can prevent the development of fibrosis by reducing bleeding into the epidural space. Similarly, TXA efficacy in preventing epidural fibrosis was demonstrated in our study. Use of systemic TXA reduces epidural bleeding and ultimately reduces epidural fibrosis. The dose of administration of the drug is as important as the route of the drug. In their recently published meta-analysis, the researchers revealed that a high dose of intravenous TXA reduced blood loss during scoliosis surgery and was no significant complication [ 31]. We would like to emphasize that the dose of TXA is as important as the route of administration. In our study, TXA dosage (30 mg/kg) was applied as Roy et al. [ 24] described in their animal study. TXA stimulates the coagulation process, by competitively preventing the conversion of plasminogen to plasmin. Therefore there are some clinical precautions and contraindications to intravenous use of TXA, such as a previous thromboembolic event, active intravascular clotting, subarachnoidal hemorrhage, and renal failure. Numerous studies submit that the administeration of intravenous TXA is successful for reducing surgical bleeding without an obvious increase in the risk of pulmonary embolism or thromboembolic complication in selected patients [ 16, 31, 32]. However, the side effects of the drug used in major surgeries such as spinal surgery should not be ignored. Serious thromboembolic adverse events were reported such as deep vein thrombosis, pulmonary embolism, cardiac arrhythmia, and myocardial infarction [ 8, 27]. Researchers have defined safe use against thromboembolic complications; caution should be exercised especially in the intravenous use of TXA. Murkin et al. [ 21] reported that the use of high-dose (initial loading dose was 100 mg/kg) TXA in older patients during cardiac surgery is associated with clinical seizures. This adverse effect could be the reduces in cerebral perfusion and blockage of inhibitory cortical γ-aminobutyric acid (GABA)-A receptors [ 21]. Antibiotics used in the postoperative period such as cephalosporins and penicillins inhibit GABA type A (GABAA) receptors and it is unknown whether these drugs aggravate the proconvulsant possessions of TXA [ 14]. Cravens et al. [ 5] reported that an unusual case of transient color vision disturbance could be ischemia after TXA administration in a patient undergoing major spine surgery. TXA is mainly excreted renally. Dose adjustment is required for patients with renal dysfunction [ 13]. Although it was determined that systemic application was more effective in our study to prevent epidural fibrosis, topical application of TXA becomes important, especially in cases where a systemic application is contraindicated. The recently published meta-analysis emphasized the effectiveness of topical TXA. That study revealed that topical application of TXA in spinal surgery was found to be effective to decrease the drainage volume and total blood loss and preserve hemoglobin levels without increasing the risk of hematoma, infection, deep venous thrombosis, and pulmonary emboli [ 17]. Similarly, Hui et al. [ 11] revealed that topical TXA use in spinal operations significantly reduces postoperative blood loss, and the hospital stay time in a recent comprehensive meta-analysis. However, there is some complication that should not be overlooked in topical application. Researchers revealed that unintended use of TXA onto the spinal cord in spinal anesthesia produces seizures [ 2, 19] and convulsion has been reported when direct application to the rat spinal canal [ 28]. These undesirable conditions bring to mind whether direct application on the nerve will have a negative effect. There is an important experimental study to answer this question. Schwarzkopf et al. [ 29] sought to examine the effect of the topical application of TXA on the sciatic nerve in rats. They exposed the sciatic nerve and then administered subtherapeutic (1 mg/kg), therapeutic (10 mg/kg), and supratherapeutic (100 mg/kg) concentrations of topical TXA. They suggested that there were no electrophysiologic and histologic effects on the sciatic nerve, for up to 1 month [ 29]. Since the spinal cord will be completely exposed after laminectomy, the dose of the TXA in the topical application becomes important; therefore, we would like to emphasize that topical application should be treated with caution. Sahin et al. [ 26] evaluated the effect of local TXA application at doses of 1, 10, and 100 mg/kg on bone healing in a spinal fusion model in rats. They showed that local use of TXA impaired the property and strength of fusion without a postponement in bone formation. They suggested that 1 mg/kg dose is a critical threshold above which TXA reduced the bone healing process of fusion. Researchers emphasized that topical TXA application in spinal surgery with fusion may adversely affect bone healing and the importance of drug dose in their studies [ 26]. Although we investigated the effects of topical and systemic TXA on epidural fibrosis formation, we could not design a study at different doses. A limitation of our study there were no different doses of TXA. This limitation was related to the number of rats we could use in this study. It may be planned in the future to evaluate whether TXA has a dose-dependent effect.
CONCLUSION
In this study, systemic and topical administration of the TXA significantly prevented the formation of epidural fibrosis. Epidural fibrosis formation was prevented more by systemic application, but the topical application was found to be effective when compared to the control group. Therefore, if there are no contraindications, we recommend the systemic and/or topical use of TXA to prevent epidural fibrosis during the spinal surgery.
Fig. 1.
Laminectomy at the L1 and L2 vertebral levels. 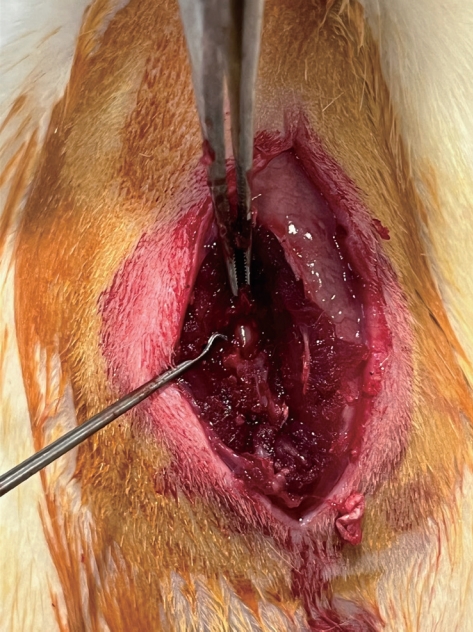
Fig. 2.
A and B : Grade 1 epidural fibrosis (EF) section from the systemic and topical tranexamic acid (TXA) group thin arrows designate the stage 3 vascular proliferation site at the interface between the dura and scar tissue. Thick arrows indicate dura mater was free of scar tissue. A residual hemostatic agent was not observed (A : Hematoxylin and Eosin [H&E], ×40; B : Masson’s trichrome, ×40). C and D : Grade 3 EF section from the control group. EF covered the whole laminectomy defect with a wide adherence to dura mater (arrows). The mass effect of a large amount of scar tissue is observed in the control group, which causes deformation of the dural tube (C : H&E, ×100; D : Masson’s trichrome, ×100). SC : spinal cord. 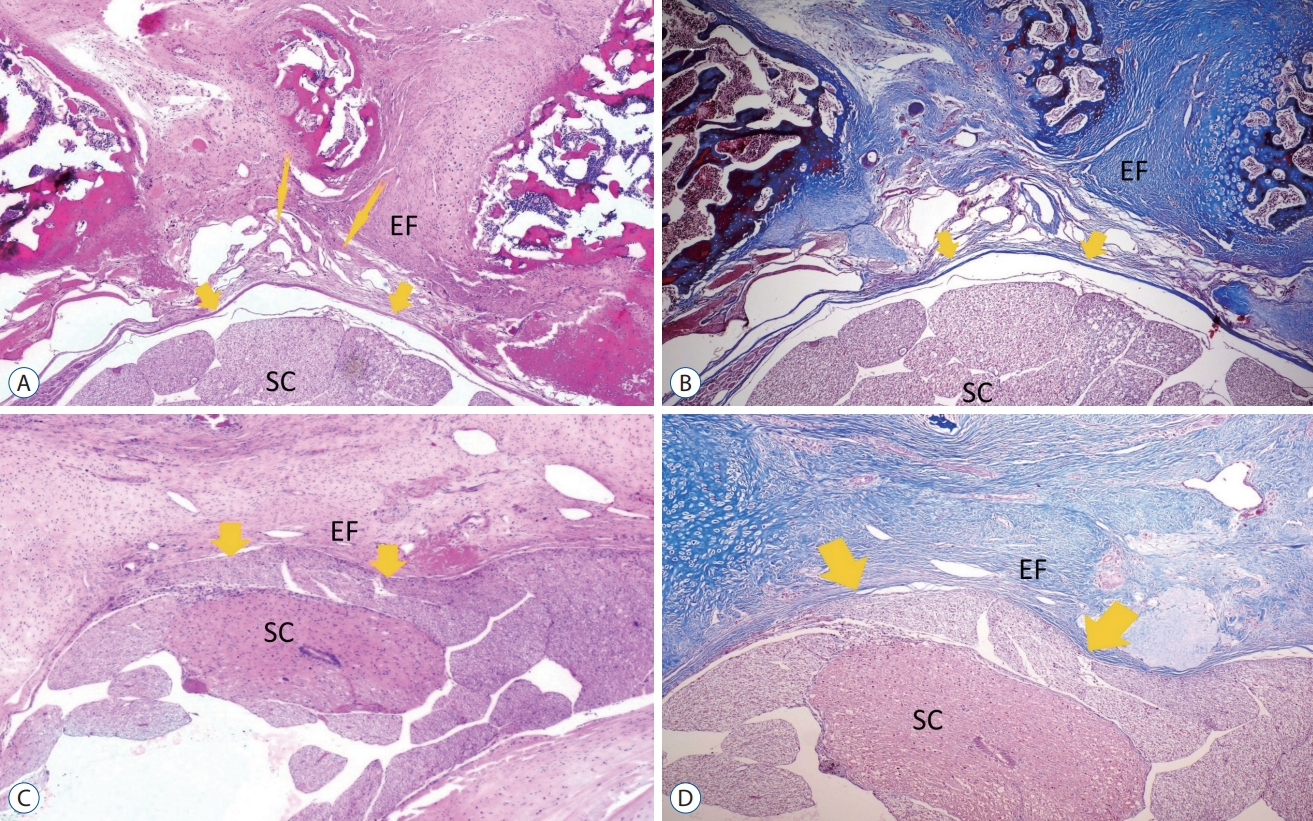
Fig. 3.
Comparison of the mean histologic sum score among groups. *Mann-Whitney U test. TXA : tranexamic acid. 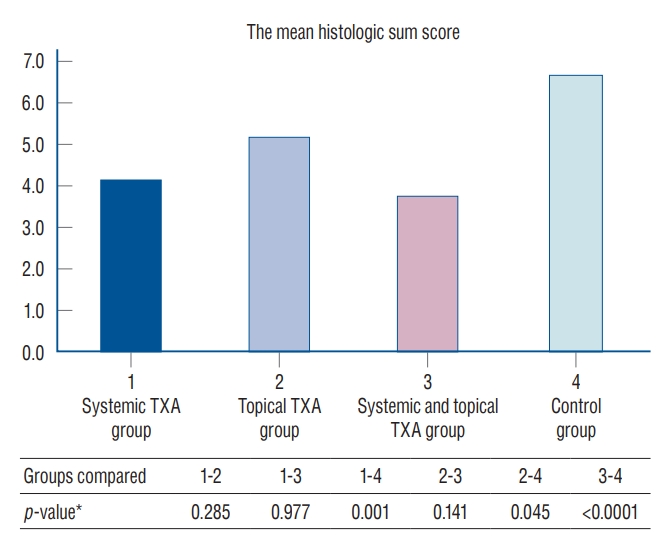
Table 1.
The mean histological grade and standard deviation, median value of the group
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
p-value |
|
Acut inflamation |
0.0 (0.0±0.0) |
0.0 (0.3±0.5) |
0.0 (0.0±0.0) |
0.5 (0.8±0.9) |
0.028*
|
|
Chronic inflamation |
1.0 (1.0±0.0) |
1.0 (1.4±0.5) |
1.0 (1.0±0.0) |
2.0 (1.8±0.7) |
0.008*
|
|
Vascular proliferation |
2.0 (1.9±0.6) |
2.0 (1.9±0.8) |
2.0 (1.9±0.8) |
2.0 (1.8±0.7) |
0.983*
|
|
Epidural fibrosis |
1.5 (1.5±0.5) |
2.0 (2.0±0.9) |
1.0 (1.3±0.5) |
3.0 (2.8±0.5) |
0.002*
|
|
Sum histologic score |
5.0 (4.4±0.9) |
5.5 (5.5±1.5) |
4 (4.1±1.1) |
7.0 (7.0±1.3) |
<0.001†
|
Table 2.
Statistical evaluation between groups
|
Groups compared |
Groups 1 and 2 |
Groups 1 and 3 |
Groups 1 and 4 |
Groups 2 and 3 |
Groups 2 and 4 |
Groups 3 and 4 |
|
Acut inflamation, p-value |
0.143 |
1.000 |
0.027*
|
0.143 |
0.223 |
0.027*
|
|
Chronic inflamation, p-value |
0.063 |
1.000 |
0.010*
|
0.063 |
0.263 |
0.010*
|
|
Vascular proliferation, p-value |
0.954 |
0.954 |
0.680 |
1.000 |
0.777 |
0.777 |
|
Epidural Fibrosis, p-value |
0.258 |
0.317 |
0.002*
|
0.079 |
0.079 |
0.001*
|
References
1. Burton CV, Kirkaldy-Willis WH, Yong-Hing K, Heithoff KB : Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res (157) : 191-199, 1981  2. Butala BP, Shah VR, Bhosale GP, Shah RB : Medication error: subarachnoid injection of tranexamic acid. Indian J Anaesth 56 : 168-170, 2012    3. Çirci E, Özalay M, Caylak B, Bacanli D, Derincek A, Tuncay IC : The effect of oophorectomy on epidural fibrosis after laminectomy: an experimental study in rats. Acta Orthop Traumatol Turc 47 : 193-200, 2013   5. Cravens GT, Brown MJ, Brown DR, Wass CT : Antifibrinolytic therapy use to mitigate blood loss during staged complex major spine surgery: postoperative visual color changes after tranexamic acid administration. Anesthesiology 105 : 1274-1276, 2006    6. Erdogan U, Tanik C, Tanriverdi O, Gunaldi O, Yilmaz I, Arslanhan A, et al : Immunohistochemical grading of epidural fibrosis with CD105 antibody. World Neurosurg 125 : e297-e303, 2019   7. Fritsch EW, Heisel J, Rupp S : The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine (Phila Pa 1976) 21 : 626-633, 1996  8. Gybel M, Kristensen K, Roseva-Nielsen N : Cardiac arrest caused by massive pulmonary embolism during treatment with tranexamic acid. Ugeskr Laeger 175 : 1426-1427, 2013  9. He Y, Revel M, Loty B : A quantitative model of post-laminectomy scar formation. Effects of a nonsteroidal anti-inflammatory drug. Spine (Phila Pa 1976) 20 : 557-563; discussion 579-580, 1995  10. Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, McClelland B, et al : Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev (1) : CD001886, 2011  12. Kasimcan MO, Bakar B, Aktaş S, Alhan A, Yilmaz M : Effectiveness of the biophysical barriers on the peridural fibrosis of a postlaminectomy rat model: an experimental research. Injury 42 : 778-781, 2011   13. Koo JR, Lee YK, Kim YS, Cho WY, Kim HK, Won NH : Acute renal cortical necrosis caused by an antifibrinolytic drug (tranexamic acid). Nephrol Dial Transplant 14 : 750-752, 1999   18. Mitchell RN, Cotran RS : Tissue repair: Cell regeneration and fibrosis. Edited by Kumar V, Cotran RS, Robbin S : In: Basic pathology. Philadelphia : W.B. Saunders, 2003, pp61-78
19. Mohseni K, Jafari A, Nobahar MR, Arami A : Polymyoclonus seizure resulting from accidental injection of tranexamic acid in spinal anesthesia. Anesth Analg 108 : 1984-1986, 2009   20. Mohsenipour I, Daniaux M, Aichner F, Twerdy K : Prevention of local scar formation after operative discectomy for lumbar disc herniation. Acta Neurochir (Wien) 140 : 9-13, 1998    21. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M : High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg 110 : 350-353, 2010   22. Robertson JT : Role of peridural fibrosis in the failed back: a review. Eur Spine J 5 Suppl 1 : S2-S6, 1996   23. Ross JS, Robertson JT, Frederickson RC, Petrie JL, Obuchowski N, Modic MT, et al : Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery 38 : 855-861; discussion 861-853, 1996  24. Roy M, Burggraf M, Lendemans S, de Groot H, Rohrig R : Tranexamic acid prolongs survival after controlled hemorrhage in rats. J Surg Res 208 : 104-110, 2017   25. Sae-Jung S, Jirarattanaphochai K, Sumananont C, Wittayapairoj K, Sukhonthamarn K : Interrater reliability of the postoperative epidural fibrosis classification: a histopathologic study in the rat model. Asian Spine J 9 : 587-594, 2015    26. Sahin E, Berk H, Ozkal S, Keskinoglu P, Balci P, Balci A : Effect of local tranexamic acid on the quality of bone healing in a rat spinal fusion model. Spine Surg Relat Res 6 : 151-158, 2022    27. Salam A, King C, Orhan O, Mak V : The great deception: tranexamic acid and extensive pulmonary emboli. BMJ Case Rep 2013 : bcr2012007808, 2013    28. Schlag MG, Hopf R, Redl H : Convulsive seizures following subdural application of fibrin sealant containing tranexamic acid in a rat model. Neurosurgery 47 : 1463-1467, 2000    29. Schwarzkopf R, Dang P, Luu M, Mozaffar T, Gupta R : Topical tranexamic acid does not affect electrophysiologic or neurovascular sciatic nerve markers in an animal model. Clin Orthop Relat Res 473 : 1074-1082, 2015    34. Zhang C, Kong X, Liu C, Liang Z, Zhao H, Tong W, et al : ERK2 small interfering RNAs prevent epidural fibrosis via the efficient inhibition of collagen expression and inflammation in laminectomy rats. Biochem Biophys Res Commun 444 : 395-400, 2014  
|
|



















