Park, Kim, and Kim: Endoscopic Treatment of Chronic Subdural Hematoma Combined with Inner Subdural Hygroma
Abstract
Objective
A chronic subdural hematoma (CSDH) is a collection of bloody fluid located in the subdural space and encapsulated by neo-membranes. An inner subdural hygroma (ISH) is observed between the inner membrane of a CSDH and the brain surface. We present six cases of CSDH combined with ISH treated via endoscopy.
Methods
Between 2011 and 2022, among the 107 patients diagnosed with CSDH in our institute, six patients were identified as presenting with CSDH combined with ISH and were included in this study. Preoperative computerized tomography (CT) and magnetic resonance imaging (MRI) were performed simultaneously, and endoscopic surgery for aspiration of the hematoma was performed in all cases of CSDH combined with ISH.
Results
The mean age of patients was 71 years (range, 66 to 79). The patients were all male. In two cases, the ISH was not identified on CT, but was clearly seen on MRI in all patients. The inner membrane of the CSDH was tense and bulging after draining of the CSDH in endoscopic view due to the high pressure of the ISH. After fenestration of the inner membrane of the CSDH and aspiration of the ISH, the membrane was sunken down due to the decreasing pressure of the ISH. There was one recurrence in post-operative 2-month follow up. The symptoms improved in all patients after surgery, and there were no surgery-related complications.
Conclusion
CSDH combined with ISH can be diagnosed on imaging, and endoscopic surgery facilitates safe and effective treatment.
Key Words: Hematoma, subdural, chronic ┬Ę Endoscopy.
INTRODUCTION
A chronic subdural hematoma (CSDH) is a collection of bloody fluid located in the subdural space and encapsulated by neo-membranes (outer and inner membranes). CSDH is a frequently encountered disease in the neurosurgical field and generally occurs in elderly patients. The overall incidence of CSDH has increased progressively to 13.5 to 20.6 per 100000 persons per year [ 1, 33]. Although the pathophysiology of CSDH is not clearly understood, angiogenesis, fibrinolysis, and inflammation are involved in the evolution of CSDH, which is presented by the formation of a membrane and hemorrhage [ 7, 11, 23, 31]. Computerized tomography (CT) is the initial diagnostic tool and provides extensive information on the radiological characteristics of CSDH. More information such as the age of the hematoma, location, contents, and internal structures might be identified by magnetic resonance imaging (MRI) [ 9, 15, 17]. CSDH is classified into several types according to its internal structure : 1) homogeneous, 2) laminar, 3) separated, and 4) trabecular [ 21]. Also, according to the internal architecture and contents of the CSDH, the recurrence rate may vary [ 25, 30]. Surgical treatment methods include twist drill craniostomy, burr-hole trephination, and craniotomy; non-surgical methods include steroid atorvastatin and tranexamic acid [ 4, 13, 28]. Although burr-hole trephination with closed system drainage is widely accepted and performed, an optimal treatment method for CSDH has not yet been established. In recent years, endoscopic treatment of CSDH has been performed in complicated and recurrent cases, and there have been many reports about the safety and efficacy of treatment of CSDH via endoscopy [ 5, 10, 12, 27]. We endoscopically observed and treated complex cases of CSDH combined with inner subdural hygroma (ISH), which were located between the inner membrane of the CSDH and the arachnoid membrane over the brain surface. We present these cases of CSDH combined with ISH and describe the clinical presentation, imaging findings, and endoscopic surgical results.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of Daegu Catholic University Medical Center (IRB No. CR-22-107-L).
Between 2011 and 2022, among 232 consecutive patients who were diagnosed with CSDH in our institution, preoperative CT and MRI were performed simultaneously in 107 patients. MRI was performed without sedation in generally and neurologically stable patients. Patients who were unable to undergo MRI, such as confused and restless patients, were excluded from this study. CSDH combined with ISH was identified mainly by MRI and could also be found on CT. Endoscopic surgery for aspiration of ISH was performed in CSDH combined with ISH because it is possible to visualize the inner membrane of CSDH via burr-hole and thus avoid wide craniotomy to manipulate the inner membrane. We retrospectively reviewed the clinical characteristics, imaging data, and operative findings in these case series.
Surgery was performed under general anesthesia. A round scalp incision was performed on the parietal region 3 cm behind the coronal suture or over the center of the CSDH. Burrhole trephination (approximately 1 cm in diameter) was performed by air drill. The cruciate dura incision was performed and the outer membrane of the CSDH was exposed. After fenestration of the outer membrane of the CSDH, which was thick and dark in color, some amount of bloody fluid from the CSDH was aspirated using a suction tube. Then, a 0┬░ or 30┬░ rigid endoscope (Karl Storz, Tuttlingen, Germany) was inserted into the space from which the CSDH had been removed. The tensive and bulging inner membrane of the CSDH, likely due to the high-pressure ISH, was identified at the base on endoscopic view. The inner membrane of the CSDH was then incised for several millimeters, and the ISH was aspirated carefully with a suction tube. After both CSDH and ISH spaces were carefully inspected by endoscope, a drain catheter was placed into the space of the CSDH with or without that of ISH. The wound was closed layer by layer. Closed system drainage was maintained for 48 to 72 hours until the drain volume was not identified. Postoperative CT scans were performed 1 week and 2 months after removal of the catheter.
RESULTS
Clinical presentations
Of the 107 patients that were diagnosed with CSDH using CT and MRI, there were six patients with CSDH combined with ISH that were included in this study. Clinical data are summarized in Table 1. The mean age of patients was 71 years (range, 66 to 79). The patients were all male. Three patients had histories of head trauma due to slipping before CSDH occurred : one patient had a fall, and one patient had a traffic accident. The sixth patient did not have any history of trauma. The mean interval period between trauma and development of CSDH was 3 weeks (range, 2 to 8). The most common symptom was headache in four patients, followed by side weakness. Five patients were on anticoagulation or antiplatelet therapy. One patient had no medication history. All patients had other medical concerns.
Imaging findings
The CSDH and ISH were divided by the inner membrane of the CSDH, and the ISH was revealed at the subdural space between the CSDH and the brain surface on CT scan and MRI ( Fig. 1). The ISH was widely spread over the brain surface but not loculated. We summarized the results of the imaging findings in Table 2. Four of six patients had trabecular type CSDH, which is defined by multiple fibrous septa in the CSDH cavity. One patient had the homogenous type, and the other patient had the separated type. Only one patient had bilateral CSDH, but ISH was found only on one side. The mean maximal width of the CSDH was 12 mm (range, 5 to 19), and the mean maximal width of the ISH was 12.8 mm (range, 6 to 20). The mean midline shifting was 7.3 mm (range, 0 to 18). ISH of two patients was not identified on CT. Among the four patients whose ISH was identified on CT, the ISH was revealed to have iso-density in two patients and low density in the other two patients. The ISH and inner membrane of the CSDH was evident and diagnosed on MRI in all patients. The ISH for all patients was homogeneous on MRI. The ISH was well revealed on MR sequences and the T2-weighted image was the best image for diagnosis, except for in one patient. Also, ISH showed well on the T1-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) sequences of MRI for diagnosis ( Fig. 2). The inner membrane of the CSDH was identified as a dark signal on the T2-weighted image.
Endoscopic findings
In all patients, after fenestration of the outer membrane of the CSDH, fluid-like and high-pressure CSDH gushed out. Although the outer membrane was very thick and dark in color, the inner membrane was thin and translucent but not clear due to the brownish color of the ISH located directly underneath ( Fig. 3A). The inner membrane of the CSDH was very tensive and bulging after aspiration of the CSDH due to the pressure of the ISH. After fenestration of the inner membrane of the CSDH and draining of the ISH, the membrane sunk down because of the decreased pressure of the ISH ( Fig. 3B). The brain surface under the arachnoid membrane showed clearly on inspection of the ISH cavity. Because there were no adhesions and internal structure like trabecular into the ISH space, the inner membrane was almost separated from the brain surface. These two spaces of the CSDH and ISH were different in nature. In one patient who had a recurrent case, the inner membrane was very thick ( Fig. 3C).
Surgical outcomes
Symptoms improved in all patients after surgery. The motor grade of the patient with side weakness improved from grade 4 to 5. Five patients recovered without recurrence; however, one patient had a recurrence of CSDH and ISH, so a second operation was performed. There were no surgery-related complications such as acute hemorrhage, brain cortex or cortical vessel injury, or postoperative infection in any patients.
Illustrative cases
Case 1
A 66-year-old man was admitted to our institute with progressive headache. The patient had experienced a traffic accident while driving due to sudden and transient vision loss. He recovered from the symptoms and had no neurological sequela, but he was diagnosed with a cerebral infarction on the right middle cerebral artery territory. An antiplatelet agent was administered for prevention of cerebral infarction. After 2 weeks, an imaging study was performed due to headache. CT showed a trabecular type CSDH on the left side. However, ISH was not clearly seen on the initial CT scan ( Fig. 4A). MRI demonstrated a CSDH on the left side and showed an ISH with compressed left hemisphere but no brain shifting ( Fig. 4B and C). Surgery for endoscopic aspiration of the CSDH and ISH was planned. A semicircular incision of the scalp was created, and burr-hole trephination was performed at the left parietal skull. After the dura mater and outer membrane of the CSDH was opened, dark fluid of the CSDH was aspirated. The tensive and bulging inner membrane of the CSDH was observed ( Fig. 5A). The inner membrane was incised by scissors via endoscope, and the ISH was aspirated. Then, the inner membrane of the CSDH sunk down, and the space left by the CSDH and ISH could be observed well on endoscope ( Fig. 5B). A 9-Fr catheter was inserted into the CSDH space and was maintained for 48 hours.
Case 2
A 71-year-old man was admitted to our institution with a severe headache. The patient had experienced a minor head trauma due to slipping 2 weeks prior to admission. He received an antiplatelet agent 4 years prior due to angina. No focal neurologic deficit was found. The laboratory values of the patient were within normal ranges. A separated type CSDH was observed on the right hemisphere on CT ( Fig. 6A), and an ISH was seen on MRI ( Fig. 6B). Surgery was performed under general anesthesia in the supine position. An incision was created on the parietal scalp, and burr-hole trephination was done. After aspiration of the CSDH fluid, the inner membrane of the CSDH was observed. The membrane was so tensive that it was almost close to the outer membrane of the CSDH on endoscopic view ( Fig. 7A). After incision of the inner membrane, brownish fluid was aspirated. Then, the inner membrane sunk down, and the spaces of CSDH and ISH could be observed widely. The CSDH space, inner membrane, ISH space, and brain surface could be inspected by endoscope ( Fig. 7B). A drainage catheter was inserted into the two spaces, and the closed system drainage was maintained for 48 hours. On follow-up CT and MRI after 2 weeks, CSDH had recurred, and ISH size had increased compared with imaging before the first operation. A second operation was done in similar fashion as the first. The inner membrane was tensive similar to the first operation. The inner membrane which had been fenestrated during the first operation, was closed with a fibrous hematoma. There was no further recurrence after the second operation.
DISCUSSION
CSDH is defined as a bloody fluid collection that compresses the brain surface and may result in symptoms. The ISH, which consists of a similar fluid collection in the subdural space, is considered to be an uncommon lesion that can co-occur with CSDH. To the best of our knowledge, CSDH combined with ISH has not yet been reported. ISH has a low incidence and can be clearly identified on MRI, which is not a routine imaging study. Because of its rarity, the number of CSDH combined with ISH cases was very small in our study, which is its main limitation. Further studies with larger numbers of cases are needed on the endoscopic treatment of the CSDH combined with ISH. Shimizu et al. [ 26] reported the isolated deep-seated hematoma, a similar lesion confirmed by intraoperative ultrasonography under the inner membrane of the CSDH and treated by fenestration of the membrane. However, the isolated deep-seated hematoma was not a lesion that was diagnosed on preoperative imaging, and the hematoma was not tensive and was asymptomatic. Furthermore, the surgery was performed only to reduce the re-expansion time of the brain but not to improve symptoms. In our study, ISH was a different lesion from isolated deep-seated hematoma. ISH was identified on preoperative imaging and surgery was necessary to diminish the mass effect and improve symptoms. Endoscopic surgery was used for treatment of ISH in our study. High-resolution imaging and inspection into the CSDH space by endoscope made it possible to identify ISHs. Although the ISH could be well revealed on CT imaging, when the density of CSDH was similar to that of the ISH, the ISH was difficult to distinguish from the CSDH. The ISH was not detected on CT imaging in two of the six patients in our study. The ISH of these patients was considered to be high density, similar to the CSDH on CT imaging. The ISH in the other four patients, on the other hand, was iso- or low density. MRI was a very valuable imaging modality for identifying ISH preoperatively; the ISH was particularly apparent on T2-weighted imaging. More information about hemorrhage according to age and CSDH contents could be obtained from other sequences of MRI [ 15]. When it is difficult to distinguish ISH from the CSDH on T2-weighted imaging, T1-weighted, FLAIR, and DWI sequences can be useful to diagnose ISH. Also, the inner membrane of the CSDH can be observed on MRI. The inner membrane was revealed as a dark signal intensity on T2-weighted imaging; this helps to define the ISH and distinguish it from the CSDH. While the trabecular type of CSDH was observed in four of the six patients, it is not possible to define the incidence of ISH according to CSDH type due to our small study population. MRI is not a standard imaging study in CSDH. Obtaining MRI is a costly and time-consuming process and has risks in unstable patients. Thus, MRI should only be performed in CSDH patients without complications, and patient selection should be considered carefully. The most common treatment method of CSDH is burr-hole aspiration, which is a widely accepted treatment modality [ 10, 16]. Recent development of endoscopy equipment and endoscopic treatment of CSDH facilitates allows for inspection of the internal structure, thereby increasing the convenience and safety of the surgery. The advantage of endoscopic treatment of CSDH is that it can visualize the CSDH space. Therefore, it is possible to remove the internal structure of CSDH easily and place a subdural catheter under endoscopic view. Many reports have discussed endoscopic treatment of CSDH. Septated CSDH, organized CSDH, recurrent CSDH, subacute subdural hematoma, and acute subdural hematoma have been reported to have been treated by endoscopy [ 3, 5, 14, 29, 32]. Trabecular type (septated type) had many fibrous septa and an old hematoma in the CSDH space [ 8]. The fibrous septa and hematoma could be removed and aspirated safely by endoscopic assistance surgery [ 5, 6, 32]. Ichimura et al.12) described inner membranotomy for the treatment of recurrent CSDH. Also, the safety and efficacy of inner membranectomy under direct vision was reported [ 2, 12, 19]. Therefore, direct visualization of the internal structure of the CSDH and inner membrane via endoscope allows for safe and efficient treatment, and fenestration of the inner membrane of the CSDH combined with ISH could be performed without any complications. In our study, there were no surgery-related complications in any patient. However, one patient experienced recurrence of CSDH and ISH. Although the inner membrane was thick and difficult to incise and fenestrate in this patient, it was safely incised and fenestrated endoscopically. However, endoscopic surgery for CSDH is still a challenging procedure and is not widely performed due to several and serious surgical complications. The surgeon should avoid injury to the brain cortex by the rigid endoscope in the subdural space during the procedure. The prolonged operation time due to set-up of the endoscope and the procedure itself is a disadvantage. Also, endoscopic surgery for recurrent CSDH or ISH can be replaced by repeated simple burr-hole aspiration. Therefore, surgeons should be aware of these considerations, and the selection of candidates and application of endoscopic surgery for CSDH should be reviewed carefully. To inspect the inner membrane of the CSDH, we first aspirated some amount of CSDH fluid, and removed the fibrous septa and old hematoma via endoscope in the trabecular type of CSDH. After confirmation of the inner membrane, which is thin and translucent but dark in color due to the hygroma under it, the inner membrane could be incised safely because it was separated from the brain surface by the hygroma. Drain catheters were placed in both the CSDH space and ISH space or in only the CSDH space under endoscopic view. Even when only one catheter was placed in one space of CSDH or ISH, hematoma fluid from both spaces could be aspirated through the opened inner membrane.
When an ISH is not diagnosed preoperatively and the operation only drains the CSDH, the volume of CSDH that was drained through the drain catheter could be insufficient, or remnant ISH on follow-up imaging after the operation could be classified as recurrent CSDH. The ISH cannot be treated by aspiration of the CSDH alone, because the CSDH is a capsulated hematoma, and the ISH is located in a different space from the CSDH. In the second operation in this situation, the inner membrane will be faced after dura opening because of bulging of the inner membrane, and the inner membrane can be mistaken for the outer membrane of the recurrent CSDH. Therefore, it is important to confirm ISH on preoperative imaging in order to perform optimal surgical treatment and to avoid a second operation. In the operating room, severe bulging and a thin and translucent but dark membrane were observed under direct vision; in the situation of an undiagnosed ISH, the inner membrane of the CSDH needs to be incised and fenestrated by endoscope. Because the ISH incurs both pressure and mass effects, it is highly likely that without removal of the ISH, the patient will not recover from their symptoms and would require a second operation.
The main source of hemorrhage of the CSDH is the outer membrane of the CSDH, which is fed from the dural vasculature [ 18]. The inner membrane of the CSDH has less vascularity due to the low reaction potential of the adjacent arachnoid membrane [ 24]. However, the inner membrane is suggested to be the contributor of hemorrhage from imaging studies about the inflammatory process and vessels in the inner membrane by evidence of an enhanced inner membrane on CT and a black band on a T2-weighted MRI [ 20, 22]. Therefore, the inner membrane of the CSDH could be considered carefully as one source of development of the ISH. However, further study is needed to identify the pathogenesis of ISH.
CONCLUSION
ISH, which is located in the subdural space between the CSDH and the brain surface, is an uncommon lesion that can appear in combination with CSDH. CSDH combined with ISH can be diagnosed on imaging study, especially MRI. The ISH presents as tensive with bulging of the inner membrane of the CSDH on endoscopic view. Endoscopic surgery can provide safe and efficient treatment. A large number of studies are needed to determine the role of endoscopic treatment and surgical results for CSDH combined with ISH.
Fig.┬Ā1.
A : Preoperative computerized tomography showing the chronic subdural hematoma (white arrow) and inner subdural hygroma (open arrow). B : The chronic subdural hematoma (white arrowhead) of trabecular type and homogeneous inner subdural hygroma (open arrowhead) on T2-weighted image. C : The inner membrane (curved arrow) between the chronic subdural hematoma and inner subdural hygroma is showed as the dark signal on the T2-weighted image. 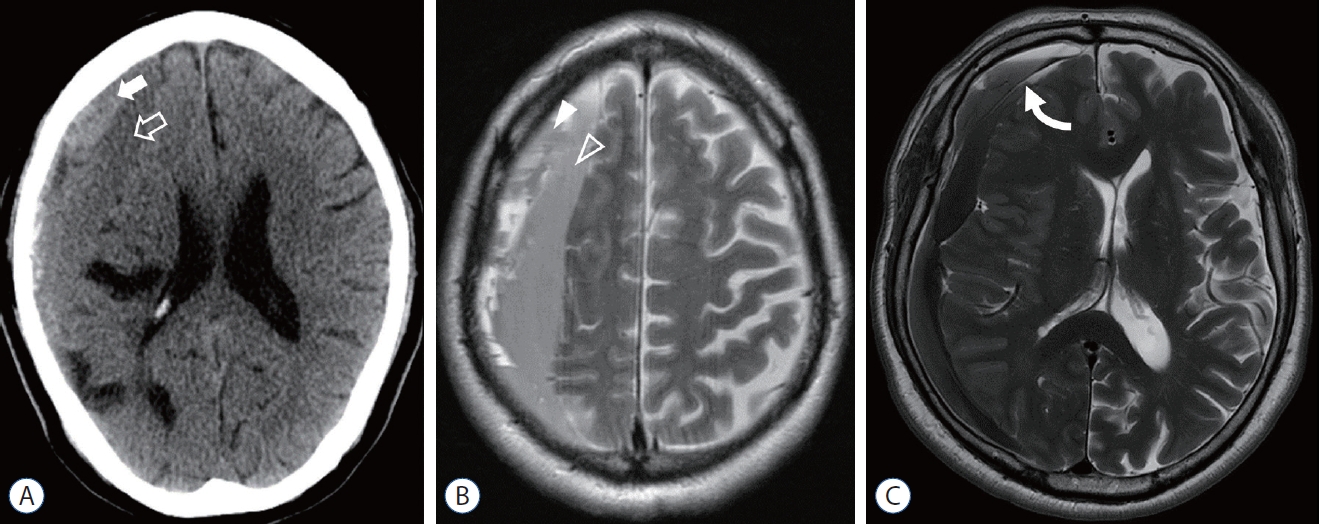
Fig.┬Ā2.
A : Preoperative computerized tomography showing the chronic subdural hematoma and inner subdural hygroma on the left side. B-F : Preoperative magnetic resonance sequences showing the chronic subdural hematoma and inner subdural hygroma; T2 weighted image (B), T1- weighted image (C), flair (D), diffusion-weighted imaging (E), and coronal T1 weighted image (F). 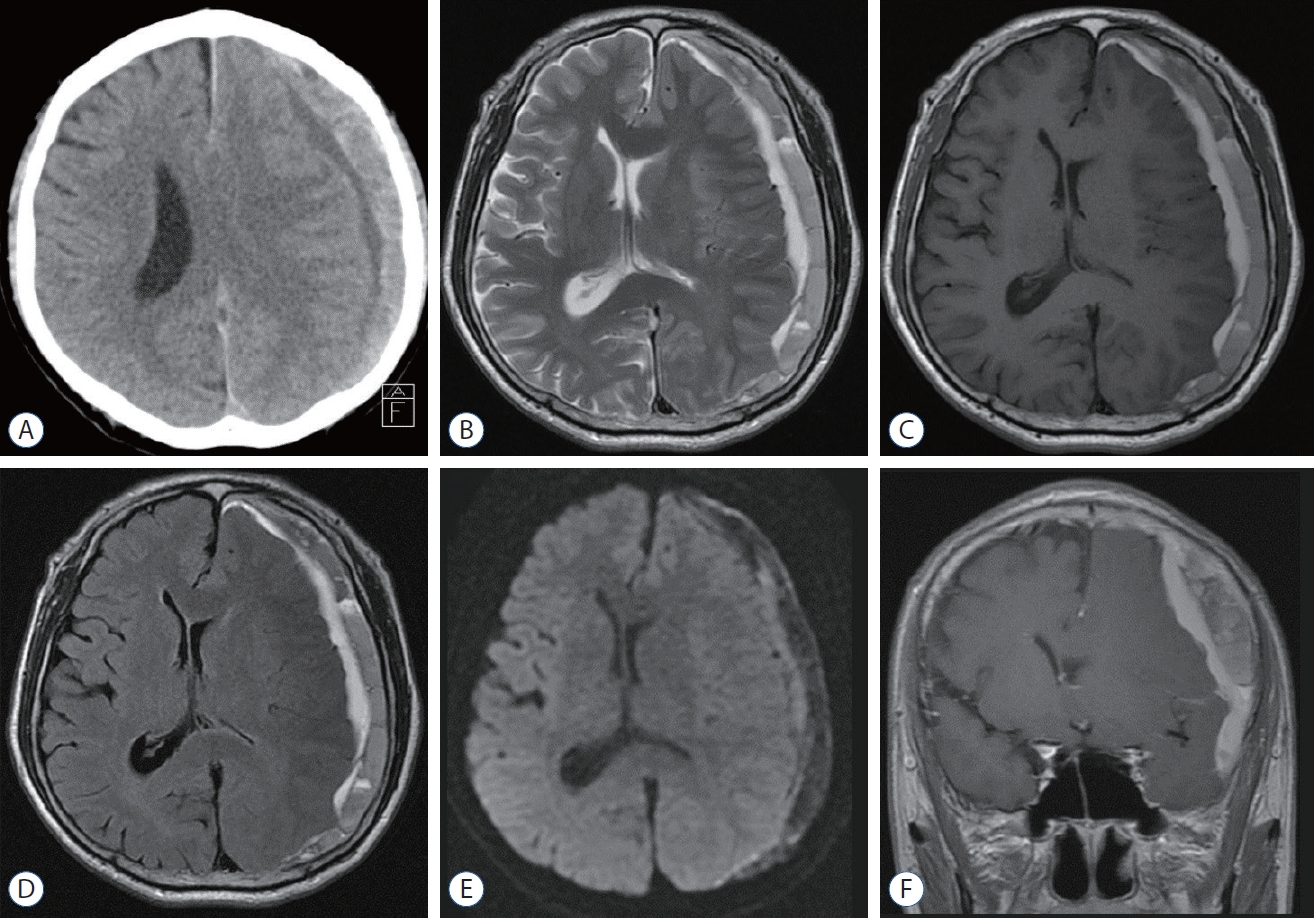
Fig.┬Ā3.
A : Endoscopic view after opening of dura and outer membrane of chronic subdural hematoma. The remnant of the outer membrane (white arrow) and the inner membrane of chronic subdural hematoma (open arrow). The inner membrane is very tense and translucent but not clear. B : After fenestration of the inner membrane and draining of the inner subdural hygroma, the inner membrane (white arrowhead) is sunken down and the brain surface (open arrowhead) was showed clearly. C : Endoscopic view of a recurrent case. The inner membrane is very thick (black asterisk). The fibrous septa (white asterisk) of trabecular type are revealed in the chronic subdural hematoma space. 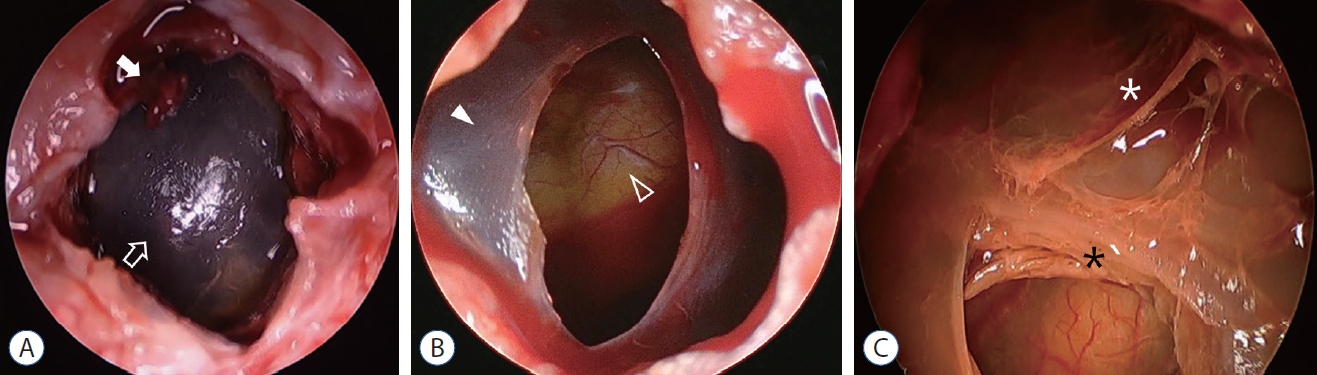
Fig.┬Ā4.
Preoperative images of illustrative case 1. A : Inner subdural hygroma is not clearly seen on computerized tomography scan. B and C : The chronic subdural hematoma and inner subdural hygroma with compressed left hemisphere is showed on T1-weighted image (B) and diffusion-weighted imaging (C). 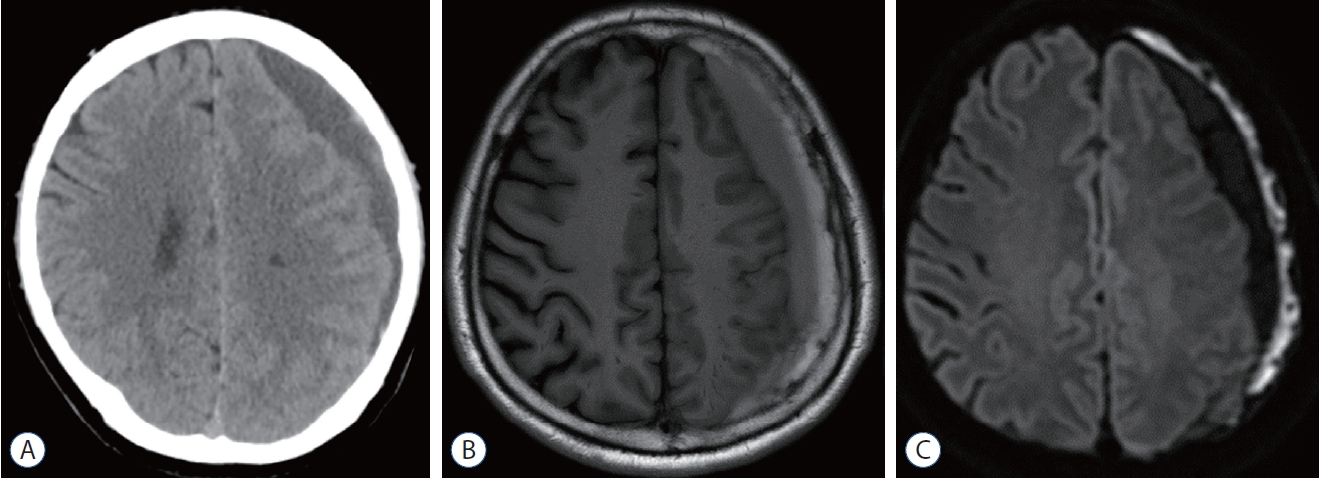
Fig.┬Ā5.
Endoscopic view of illustrative case 1. A : The bulging inner membrane of the chronic subdural hematoma (open arrow) and narrowed chronic subdural hematoma space (white arrow). B : After fenestration of the inner membrane, the chronic subdural hematoma space is widened (white arrowhead) and the brain surface (open arrowhead) is showing via the opening of the inner membrane. 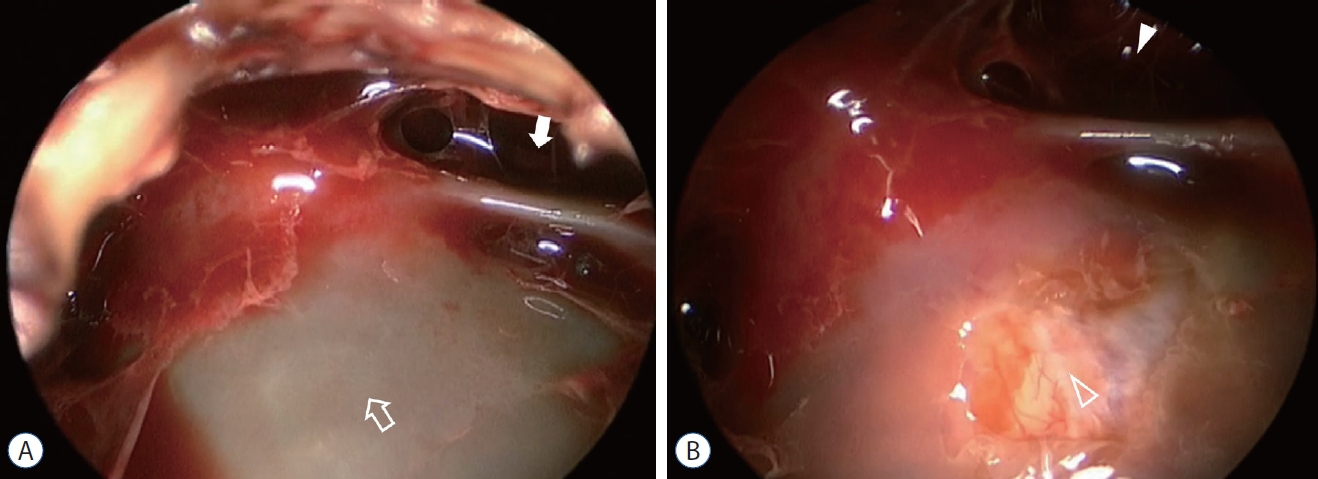
Fig.┬Ā6.
Preoperative image of illustrative case 2. A : The separated type of chronic subdural hematoma on the right-side hemisphere is demonstrated on computerized tomography, however, inner subdural hygroma is not identified. B : The inner subdural hygroma is revealed well on magnetic resonance imaging. 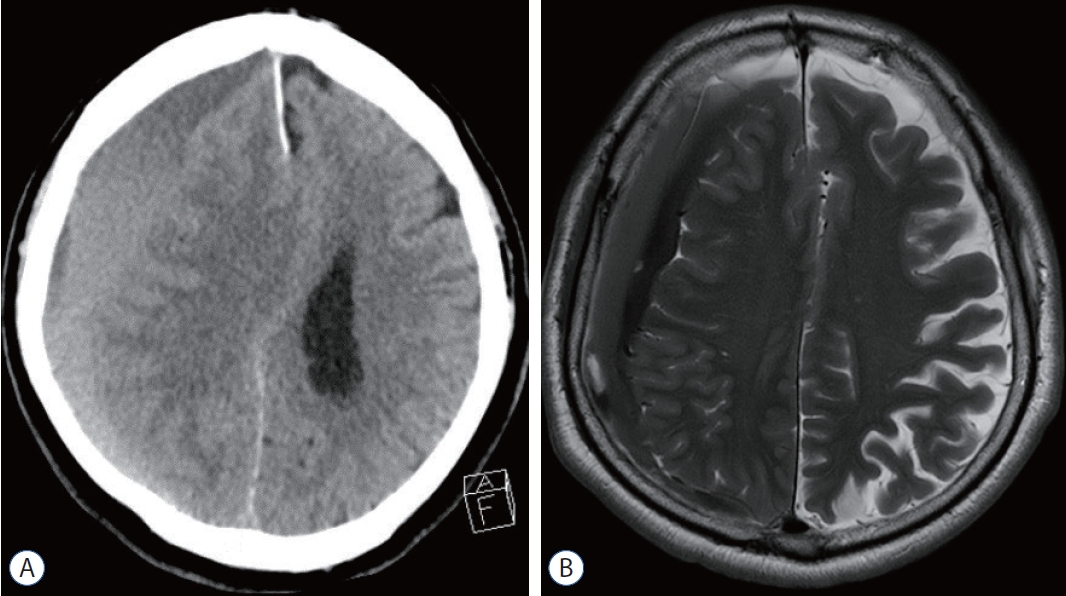
Fig.┬Ā7.
Endoscopic view of illustrative case 2. A : The inner membrane is tense and gray (open arrow), and the chronic subdural hematoma space is narrowed (white arrow). B : After incision of the inner membrane (white asterisk), the inner membrane is sunken down, and the spaces of chronic subdural hematoma and inner subdural hygroma is observed widely. The remnant chronic subdural hematoma (white arrowhead) is also observed. 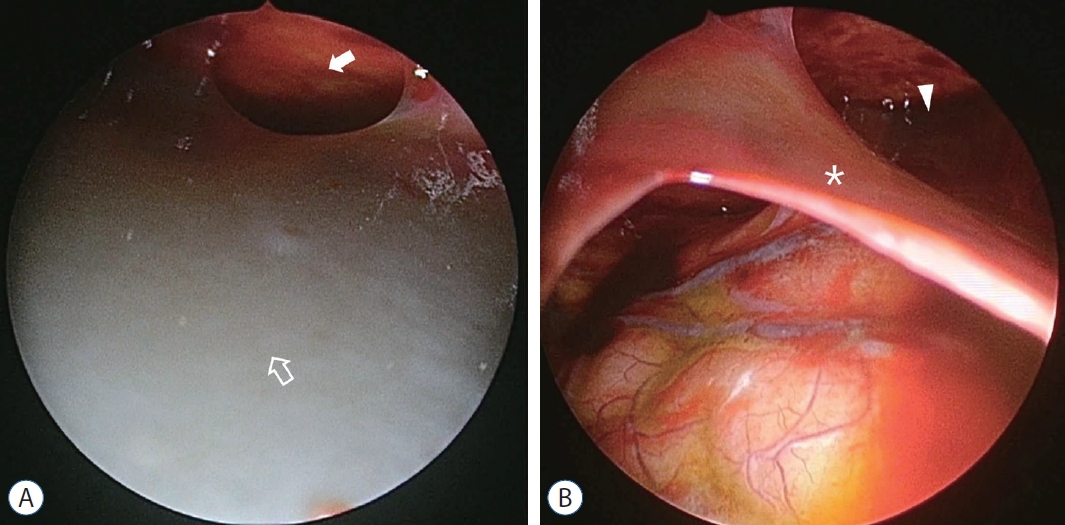
Table┬Ā1.
Clinical data of 6 patients with CSDH combined with ISH
|
Case No. |
Age (years) |
Sex |
TBI history |
Between TBI and SDH |
Symptom |
Drug history |
Past history |
|
1 |
66 |
M |
Slipping |
8 weeks |
Headache |
Aspirin |
CKD, CVA |
|
2 |
67 |
M |
None |
- |
Weakness, seizure |
Clopidogrel |
HTN |
|
3 |
79 |
M |
Slipping |
2 weeks |
Headache, weakness |
Pradaxa |
HTN, CVA |
|
4 |
71 |
M |
Slipping |
3 weeks |
Headache |
Aspirin, plavix |
HTN, DM, angina |
|
5 |
66 |
M |
Traffic accident |
2 weeks |
Headache |
Aspirin, clopidogrel |
HTN |
|
6 |
77 |
M |
Fall |
3 weeks |
Mental change, weakness |
None |
HTN, DM, HCC |
Table┬Ā2.
Results of the imaging findings in 6 patients with CSDH combined with ISH
|
Case No. |
CSDH type |
Width of CSDH (mm) |
Width of ISH (mm) |
Midline shifting (mm) |
ISH on CT
|
Identification of ISH on MRI |
|
Identification |
Density |
|
1 |
Trabecular |
12 |
19 |
3 |
+ |
Iso |
T2, FLAIR |
|
2 |
Trabecular |
13 |
6 |
13 |
+ |
Low |
T2, T1, FLAIR |
|
3 |
Homogeneous |
14 |
13 |
2 |
+ |
Iso |
T2, FLAIR, DWI |
|
4 |
Separated |
19 |
20 |
18 |
- |
- |
T2, FLAIR |
|
5 |
Trabecular |
14 |
10 |
6 |
- |
- |
T1, FLAIR, DWI |
|
6 |
Trabecular |
5 |
11 |
8 |
+ |
Low |
T2, FLAIR |
References
1. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al : Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg 259 : 449-457, 2014  2. Berhouma M, Jacquesson T, Jouanneau E : The minimally invasive endoscopic management of septated chronic subdural hematomas: surgical technique. Acta Neurochir (Wien) 156 : 2359-2362, 2014    3. Cai Q, Guo Q, Zhang F, Sun D, Zhang W, Ji B, et al : Evacuation of chronic and subacute subdural hematoma via transcranial neuroendoscopic approach. Neuropsychiatr Dis Treat 15 : 385-390, 2019   4. Chan DY, Chan DT, Sun TF, Ng SC, Wong GK, Poon WS : The use of atorvastatin for chronic subdural haematoma: a retrospective cohort comparison study. Br J Neurosurg 31 : 72-77, 2017    5. Deng J, Wang F, Wang H, Zhao M, Chen G, Shangguan H, et al : Efficacy of neuroendoscopic treatment for septated chronic subdural hematoma. Front Neurol 12 : 765109, 2021    6. Du B, Xu J, Hu J, Zhong X, Liang J, Lei P, et al : A clinical study of the intra-neuroendoscopic technique for the treatment of subacute-chronic and chronic septal subdural hematoma. Front Neurol 10 : 1408, 2020    8. Fan G, Ding J, Wang H, Wang Y, Liu Y, Wang C, et al : Risk factors for the development of chronic subdural hematoma in patients with subdural hygroma. Br J Neurosurg 35 : 1-6, 2021   9. Goto H, Ishikawa O, Nomura M, Tanaka K, Nomura S, Maeda K : Magnetic resonance imaging findings predict the recurrence of chronic subdural hematoma. Neurol Med Chir (Tokyo) 55 : 173-178, 2015    10. Guo S, Gao W, Cheng W, Liang C, Wu A : Endoscope-assisted surgery vs. burr-hole craniostomy for the treatment of chronic subdural hematoma: a systemic review and meta-analysis. Front Neurol 11 : 540911, 2020    11. Hohenstein A, Erber R, Schilling L, Weigel R : Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma 22 : 518-528, 2005   12. Ichimura S, Takahara K, Nakaya M, Yoshida K, Fujii K : Neuroendoscopic technique for recurrent chronic subdural hematoma with small craniotomy. Turk Neurosurg 30 : 701-706, 2020  13. Kageyama H, Toyooka T, Tsuzuki N, Oka K : Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg 119 : 332-337, 2013   14. Kawasaki T, Kurosaki Y, Fukuda H, Kinosada M, Ishibashi R, Handa A, et al : Flexible endoscopically assisted evacuation of acute and subacute subdural hematoma through a small craniotomy: preliminary results. Acta Neurochir (Wien) 160 : 241-248, 2018    18. Lin GX, Chen CM, Kim JS, Song KS : The transformation of intracranial subdural hygroma to chronic subdural hematoma following endoscopic spinal surgery: a case report. J Neurol Surg A Cent Eur Neurosurg 83 : 502-506, 2022   19. Mobbs R, Khong P : Endoscopic-assisted evacuation of subdural collections. J Clin Neurosci 16 : 701-704, 2009   20. Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al : Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role of thrombomodulin in the mechanism. J Neurosurg 96 : 877-884, 2002   21. Nakaguchi H, Tanishima T, Yoshimasu N : Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg 95 : 256-262, 2001   22. Nakaguchi H, Yoshimasu N, Tanishima T : Relationship between the natural history of chronic subdural hematoma and enhancement of the inner membrane on post-contrast CT scan. No Shinkei Geka 31 : 157-164, 2003  23. Pripp AH, Stani┼Īi─ć M : The correlation between pro- and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS One 9 : e90149, 2014    24. Sato S, Suzuki J : Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg 43 : 569-578, 1975   25. Sherrod BA, Baker C, Gamboa N, McNally S, Grandhi R : Preoperative MRI characteristics predict chronic subdural haematoma postoperative recurrence: a meta-analysis. Br J Neurosurg 35 : 527-531, 2021   26. Shimizu S, Mochizuki T, Osawa S, Kumabe T : Intraoperative ultrasonography during drainage for chronic subdural hematomas: a technique to release isolated deep-seated hematomas--technical note. Neurol Med Chir (Tokyo) 55 : 761-765, 2015    27. Singh H, Patir R, Vaishya S, Miglani R, Gupta A, Kaur A : Endoscopic evacuation of septated chronic subdural hemorrhage - technical considerations, results, and outcome. Surg Neurol Int 13 : 8, 2022    28. Sun TF, Boet R, Poon WS : Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg 19 : 327-333, 2005   29. Takahashi S, Yazaki T, Nitori N, Kano T, Yoshida K, Kawase T : Neuroendoscope-assisted removal of an organized chronic subdural hematoma in a patient on bevacizumab therapy--case report. Neurol Med Chir (Tokyo) 51 : 515-518, 2011   30. Tsutsumi K, Maeda K, Iijima A, Usui M, Okada Y, Kirino T : The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg 87 : 870-875, 1997   31. Weir B, Gordon P : Factors affecting coagulation: fibrinolysis in chronic subdural fluid collections. J Neurosurg 58 : 242-245, 1983   32. Yan K, Gao H, Zhou X, Wu W, Xu W, Xu Y, et al : A retrospective analysis of postoperative recurrence of septated chronic subdural haematoma: endoscopic surgery versus burr hole craniotomy. Neurol Res 39 : 803-812, 2017   33. Yang W, Huang J : Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am 28 : 205-210, 2017 
|
|























