Liu, Huang, Zhang, Chen, Zheng, Shen, Tang, Yang, and Li: Differences in Clinical Characteristics and Surgical Outcomes of Patients with Ischemic and Hemorrhagic Pituitary Adenomas
Abstract
Objective
Ischemia and hemorrhage of pituitary adenomas (PA) caused important clinical syndrome. However, the differences on clinical characteristics and surgical outcomes between these two kinds apoplexy were less reported.
Methods
A retrospective analysis was made of patients with pituitary apoplexy between January 2013 and June 2018. Baseline and clinical characteristics before surgery were reviewed. All patients underwent transsphenoidal surgery and were followed up at least 1 year.
Results
Total 67 cases (5.8%) among 1147 pituitary tumor patients were enrolled, which consisted of 28 (~2.4%) ischemic PA and 39 (~3.4%) hemorrhagic PA. There were more male patients in the ischemic group compared with hemorrhagic group (78.6% vs 53.8%, p=0.043). However, the mean age, tumor size and functional tumor ratio were significant higher in the hemorrhagic group. Headache was more common in ischemic PA (82.1%) than that of hemorrhagic PA (51.3%, p=0.011). Magnetic resonance imaging findings found that mucosal thickening and enhancement of the sphenoid sinus was observed in 15 ischemic PA patients (n=27, 55.6%), but none in patients with hemorrhagic PA (n=38, p<0.0001). It was worth noting that the rate of pre-surgical hypopituitarism in ischemic PA patients were seemed higher than that in hemorrhagic PA patients, but not significant. The two groups got a total tumor resection rate at 94.1% and 92.9%, independently. No significant difference on the operative time, blood loss in operation and complications in perioperative period was observed in two groups. After operation, cranial nerve symptoms recovered to normal at 81.8% of ischemic PA patients and 82.6% of hemorrhagic PA patients. Importantly, the incidence of postoperative hypopituitarism partially decreased in both groups, among which the rate of hypothyroidism in ischemic PA patients significantly decreased from 46.4% to 18.5% (p=0.044).
Conclusion
Patients with ischemic PA presented different clinical characteristics to the hemorrhagic ones. Transsphenoidal surgery should be considered for the patients with neuro-ophthalmic deficits and might benefit for pituitary function recovery of the apoplectic adenoma patients, especially pituitary thyroid axis in ischemic PA patients.
Key Words: Pituitary adenomas · Hemorrhage · Ischemia · Neurological disorders · Pituitary hormones.
INTRODUCTION
Pituitary apoplexy is an emergency caused by the ischemia or hemorrhage of pituitary adenomas (PA) and presents with sudden onset of headache, vomiting, visual disturbances, cranial nerves (III, IV, and VI) paresis, and hypopituitarism [ 13, 14]. It has been reported that pituitary apoplexy occurs in 2-12% of PA patients and the prevalence in non-functioning adenomas and prolactinomas is slightly higher than other subtypes [ 16, 18]. Systemic hypertension, major surgery, dynamic pituitary function tests, anticoagulation therapy, initiation or withdrawal of dopamine receptor agonists, radiation therapy, etc. are regarded as precipitating factors of pituitary apoplexy [ 14]. Patients with ischemic and hemorrhagic PA firstly suggested be estimated whether medically with steroid replacement or not, and then conservative treatment or surgical intervention should be made carefully by a multidisciplinary team. Several researches supported the evidence that surgery is recommended for the neurological recovery, but uncertain for the recovery of pituitary function [ 5, 8, 14]. Because of the similar clinical manifestations, previous studies more likely put the ischemic and hemorrhagic PA together to discuss. However, there were rare research on illustrating the differences between ischemic and hemorrhagic PA. In operation, the tumor cavity of hemorrhagic PA are filled with typical adenoma tissues companied with mass or minor hematomas, while yellow-gray or yellow-white silt like mass tumor tissues are observed in ischemic PA. Considering the different etiology of the two kinds apoplexy and different pathophysiological factors contributing to the development of ischemic and hemorrhagic PA, whether the clinical characters and outcomes are diverse needed further study to clarify. In the current study, we retrospectively analyzed a total 67 patients with pituitary apoplexy (including 28 ischemic PA and 39 hemorrhagic PA) who received transsphenoidal surgery in our department to summary the main characteristics of clinical features, endocrine manifestations, neuroimaging manifestations, and prognosis.
MATERIALS AND METHODS
The collection procedure of patient tissue samples in this study was approved by Laboratory Animal Welfare and Ethics Committee of Xinqiao Hospital (the ethical review number : 2022-356-01).
Patients
All 67 cases diagnosed with PA apoplexy were selected from our pituitary tumor treat group between January 2013 and December 2018. All the patients were suffered with onset of clinical neurological and neuroendocrinal symptoms (including headache, vision impairment, nausea/vomiting, weakness, etc.) and confirmed with pituitary tumor mass by computed tomography/magnetic resonance imaging (MRI) imaging. Differential diagnosis of ischemic and hemorrhagic PA was defined by intraoperative findings and postoperative histopathology. For the diagnosis of ischemic PA [ 16, 19, 21], yellowpink or yellow-white coagulative necrotic tumor tissues with poor or no blood supply are observed during operation, then histopathological staining found that large areas of pink, acellular, coagulative necrosis are observed in tumor samples ( Fig. 1). For the diagnosis of hemorrhagic PA [ 20], typical adenoma tissues with abundant blood supply were filled with blood clot or dark red bloody fluid in operation, then histopathological staining found that typical tumor cells surrounded by red blood cells ( Fig. 2). Nine patients with both operative and histopathological characters of ischemic and hemorrhagic PA were identified as hemorrhagic infarction and excluded.
Clinical assessment
Pituitary function was assessed by the local endocrinologists, and plasma biomarkers were measured at per-operation, postoperation and long-term following including plasma cortisol, adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4), follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone, testosterone (T), estradiol (E2), prolactin, growth hormone (GH), insulin-like growth factor 1 (IGF-1). Hypocortisolism was defined by a morning serum (8:00 am) cortisol level below 101.2 nmol/L. Central hypothyroidism was defined by a FT4 level below 11 pmol/L, with normal or low TSH level. Central hypogonadotropism was defined by a T level in male below 0.38 nmol/L and E2 level in female below 21 pg/mL, with normal or low FSH/LH level. Hypopituitarism was diagnosed when two or more hormone deficiencies could be documented. All hormones were measured on a commercially available automated immunoassay platform. All subjects underwent neuro-ophthalmic assessment (for loss of visual acuity, visual field defect and ocular paresis) when they were at admission.
Treatment
All our patients underwent transsphenoidal surgery with microscope and endoscope by experienced neurosurgeons. The excisions were sent for further histopathologically examined by Hematoxylin and Eosin (H&E) staining and immunohistochemical staining. Preoperative hypopituitarism was determined by endocrine measurements, and empirical hormone replacement of glucocorticoids was performed for patients with signs of hypocortisolism before surgery, and levothyroxine sodium tablets were administered every day preoperatively for patients with hypothyroidism. Hydrocortisone (100 mg, intravenously) were administered at 30 minutes before operation for routine preparation for transsphenoidal surgery.
Follow‑up
After surgery, all patients were followed up at 1, 3, 6 months, 1 year and long-term, which was the latest one especially for this study. The follow-up evaluations included assessment of endocrinological findings and sellar MRI findings.
Statistical analysis
Independent-samples t-test was carried out for statistically analyzing the continuous variables, and chi-square test was for categorical variables by using SPSS version 18.0 (IBM, New York, NY, USA). Data were expressed as mean±standard deviation, and p<0.05 was defined to be significantly.
RESULTS
According to our definition, we identified 67 cases (5.8%) among 1147 pituitary tumor patients over a 6-year period (2013-2018), which consisted of 28 (~2.4%) ischemic PA and 39 (~3.4%) hemorrhagic PA. Age range was 19 to 77 years (ischemic PA, 19-73 years; mean, 43.4±2.9 years; hemorrhagic PA, 26-77 years; mean, 51.4±2.0 years; p=0.02). Among the 67 patients, 24 (35.8%) were female where there were six in ischemic PA cohort and 18 in hemorrhagic PA cohort (p=0.043). Basal hormone levels of all 67 patients, including TSH, FT3, FT4, LH, FSH, E2, T, GH, IGF-1, ACTH and cortisol, had been assessed when they were on admission. Of the 28 patients with ischemic PA, six (21.4%) had been previously diagnosed with functioning pituitary adenoma (FPA), whereas 22 patients (56.4%, p=0.006) in hemorrhagic PA (n=39) were aware of the presence of a FPA. In hemorrhagic PA patients, 48.7% were diagnosed with complete anterior pituitary insufficiency before operation. Compared with hemorrhagic PA cohort, ischemic PA patients seemed more likely to suffer pituitary insufficiency (60.7%), but there have no differences between them ( p=0.457, Table 1). Briefly, headache was the leading symptom of ischemic and hemorrhagic PA, which was more common in ischemic PA (82.1%) than that of hemorrhagic PA (51.3%, p=0.011). The incidence rate of other common apoplectic symptoms (including weakness, nausea, vomiting) was similar between ischemic and hemorrhagic PA patients. The rate of hyponatremia was higher in ischemic PA patients (51.8%) compared with hemorrhagic patients (27.8%), but not significant. Forty patients (59.7%) complained of visual field impairment, and diplopia, in ischemic (14 patients) and hemorrhagic (26 patients) PA cohort (p=0.211). Limited eye movements including the oculomotor nerve (the third cranial nerve) palsies, the trochlear nerve (forth cranial nerve), and abducens nerve (sixth cranial nerve) palsies, presented in 34 patients. Although those symptoms in hemorrhagic PA patients (56.4%) seemed to be higher than that (42.9%) of ischemic PA patients, this didn’t reach statistic point.
All patients performed with MRI examinations except one who was limited by cardiac valve replacement. Patients with hemorrhagic PA had significantly larger tumors in axial, coronal and sagittal planes (2.9×2.1×2.8 cm vs. 2.4×1.9×2.5 cm; p=0.012). And mucosal thickening and enhancement of the sphenoid sinus was observed in 15 (n=27, 55.6%) ischemic PA patients ( Fig. 1B and C), but none in patients with hemorrhagic PA (n=38, p<0.0001). Pituitary ring sign were reported to be strong predictors for pituitary apoplexy [ 18]. In our study, we found 25.9% ischemic PA patients exist, which seemed to be higher than that of hemorrhagic PA patients (17.1%), but no statistical significance. All 67 patients underwent transsphenoidal surgery as primary treatment. The average operative time were 69.3±6.9 minutes for ischemic PA and 82.3±8.0 minutes for hemorrhagic PA. The mean blood loss in operation of ischemic PA and hemorrhagic PA were 97.3±16.9 mL and 192.9±62.5 mL. There were no any statistically significant differences in operative time and blood loss between ischemic PA and hemorrhagic PA. The hospital stays of ischemic PA (15.0±1.3) were similar with hemorrhagic PA (14.6±0.9) ( Table 2). The temporary diabetes insipidus were observed in 13 (46.4%) ischemic PA patients and 19 (48.7%) hemorrhagic PA patients ( p=1.00), but no permanent diabetes insipidus was present in any patient postoperatively. 25.0% patients with ischemic PA and 30.8% patients with hemorrhagic PA presented with cerebrospinal leakage after operation, but there were not significant. Totally three patients underwent bacterial meningitis, including two ischemic and one hemorrhagic PA patients, all were cured with antibiotic. 94.1% of ischemic PA patients and 92.9% of hemorrhagic PA patients endured total tumor resection. After operations, 69.2% of ischemic PA patients and 85.7% of hemorrhagic PA patients had visual improvement ( p=0.237). At the last follow-up, the cranial nerve palsy of 11 ischemic PA patients (81.8%) and 19 hemorrhagic PA patients (82.6%) had recovered to normal. Hydrocortisone (20 or 40 mg) was administered in oral at 8:00 am and 16:00 pm for patients with ACTH deficiency, and levothyroxine sodium tablets (25-75 μg) were administered every day preoperatively for patients with hypothyroidism. The dosages of hydrocortisone and levothyroxine had no differences before discharge ( Table 2). Hormonal pituitary evaluations are mandatory to diagnose hypopituitarism, ischemic PA patients be more likely to had hypocortisolemia (46.4%), but there had no difference with hemorrhagic PA patients before operation (35.9%, p=0.453). Hypothyroidism was found to be more common in ischemic PA patients, occurring in 46.4% of patients, but 28.2% in hemorrhagic PA patients ( p=0.196). And hypogonadotropism occurred in 64.3% of ischemic PA patients and 48.8% of hemorrhagic PA patients ( p=0.225). After operation, we followed endocrine levels. At the last follow-up, 37.0% ischemic PA patients and 35.9% hemorrhagic PA patients remained cortisol deficiency. 18.5% ischemic PA patients and 19.4% hemorrhagic PA patients presented hypothyroidism. Intriguingly, the incidence of hypothyroidism in ischemic PA patients was significantly decreased after surgery ( p=0.044), but not in hemorrhagic group, which supported a positive role of surgery in the recovery of pituitary thyroid axis. 40.7% of ischemic PA patients and 33.3% hemorrhagic PA patients were gonadotrophic deficiencies ( Table 3).
DISCUSSION
Pituitary apoplexy occurs as a rare emergency in neurosurgery patients but in a significant number of patients with PA. Intriguingly, the incidence of apoplexy in PA is significantly higher than the other CNS tumors [ 4, 12]. In the past decades, there were several researches on the hemorrhagic PA, however, little attentions have been paid on the different characteristics between the ischemic and hemorrhagic PA. In the current retrospective study, a total of surgical 27 ischemic PA patients and 39 hemorrhagic PA patients were analyzed. We found that there were several discrepancies on clinical manifestations, endocrinological functions and surgical outcomes between the two kinds of PA, which provided us more awareness on PA. During our research period from 2013 to 2018, PA occurred in 5.8% of all 1147 PA patients, which was similar to the previous reports [ 6, 14, 18]. Limited literatures have illustrated the different occurrence rate of the two subtypes PA. Ogawa et al. [ 11] reported ischemic apoplexy occurred in 1.5% of 1067 PA patients in Japan, while 2.53% hemorrhagic cases. In Chinese people, Xiao et al. [ 19] concluded an occurrence rate ischemic PA at 2.18% (9/412) of PA patients. Recently, another group reported a total rate at 8.6% of experienced apoplexy in a larger series cases with 5095 patients and found a subtype of infarctive PA, which named coagulative necrotic pituitary apoplexy, with an occurrence rate at 4.8% [ 16]. Sixty-seven cases (28 ischemic ones and 39 hemorrhagic ones) with follow-up were enrolled in the study. In our study, the mean age of ischemic PA group was younger. The rate of male patients in ischemic PA group was significantly higher than that in hemorrhagic PA group (78.6% vs. 53.8%), which was consistent with previous study on men with a high morbidity [ 11, 16]. The mean volume of patients with ischemic PA was dramatically smaller on MRI, and this could be explained by acute intratumoral hemorrhage inducing the volume enlarged in hemorrhagic PA patients. In ischemic PA group, patients with nonfunctioning adenomas significantly outnumbered patients with functional PAs, which was similar to the reported features of ischemic PA [ 16], but contrary to Xiao et al.’s findings [ 19]. Considering the decrease of hormones secretion induced by necrosis of tumor cells, the hypersecretion of functional tumor might be affected by the duration. Moreover, as there is commonly clinical unawareness of a preexisting pituitary tumor before tumor apoplexy, the functional diagnosis may be difficult and delayed with a wide differential diagnosis [ 14]. There were several potential intrinsic and extrinsic pathophysiological mechanisms on the progress of PA [ 4, 7, 10, 12]. The key different pathological process of ischemic and hemorrhagic PA is whether the PA undergoes hemorrhage or infarction, which might present different symptoms. We found that sudden headache was the leading symptom of patients with ischemic PA, and was more common than hemorrhagic group. This symptom was similar with aseptic meningitis and might be explained by stimulation of meninges by necrotic cellular compounds [ 11]. Acute vision loss was the leading symptom of patients with hemorrhagic PA, and was slight higher than ischemic group but not significant. This might seem not in accordance with the larger volume of hemorrhagic tumors. Besides of secondary edema of necrotizing tissue compression to the optic nerves, another possible pathophysiological mechanism of infarction is direct compression of portal vessels or the hypophyseal arteries by tumor mass [ 16]. As we known, optic chiasma was partially supplied by branches of superior hypophyseal arterys [ 17]. Thus might be a reason of infarctive PA induced vision loss. However, the impairment of cranial nerves III, IV, and VI seemed to be caused by progressive intracavernous invasion of the tumor mass and pressure increases in the pituitary region partially. In our study, we found the average volume of tumor in hemorrhagic group was larger than that of ischemic group. And surgical decompression could improve the symptom of neuropathy. Thus, we are inclined to believe that mechanical compression mechanism may result in nerve dysfunction of PA patients. Multiple adenohypophysis hormonal deficiencies induced endocrine dysfunctions were commonly observed in patients with PA [ 4, 13, 14]. Nausea, vomiting and weakness may occur as a result of adrenal insufficiency [ 4]. In our study, the incidence of neuroendocrine symptoms (mainly including nausea, vomiting, weakness, hyponatremia) of ischemic PA patients were similar with that of hemorrhagic group. The anterior pituitary deficiency at the onset of PA was common in both groups. Maybe that is due to the progressive tumor mass effect on the normal pituitary gland or the reduced blood provision to pituitary, but we failure to find differences between ischemic PA patients and hemorrhagic group. In our study, corticotropic, thyrotropic and gonadotropic deficiencies have been observed in 46.4%, 46.4%, and 64.3%, which are similar to previous studies. MRI is well recommended for the diagnosis of PA [ 3]. The phenomenon of sphenoid sinus mucosa thickening (SSMT) during the acute phase of PA was first described in 2001 [ 2]. Waqar et al. [ 17] found the incidence of SSMT was higher in patients with unclassified PA than that in control group of surgically treated non-functioning PAs. The SSMT sign might precede an apoplectic event of pituitary tumor [ 1] and the PA patients with SSMT sign might present severer neurological/endocrinological symptoms and poor prognosis [ 9]. Wang et al. [ 16] found that SSMT was observed in 11/21 patients with ischemic PA. In our study, we found that SSMT presented in 55.6% of ischemic PA patients, but none in patients with hemorrhagic PA, which suggested a unique neuroimaging finding of ischemic PA. However, the etiology of SSMT in the ischemic PA remains unclear and inf lammatory reaction might play a potential role in the development of SSMT [ 1, 2]. Another MRI feature in patients with ischemic PA was named “pituitary ring sign” in 2007 [ 15], which referred tumoral peripheral enhancement surrounding a hypointense area on T1-weighted contrast-enhanced MRI. The supposed etiology of the pituitary ring sign is the hypointense area in the tumor is necrotic tissues, which is not enhanced by gadolinium, and the enhanced part of the tumor is the outer portion of pituitary or its “skin”. However, considering the signal of blood clots in MRI changing over time, the MRI imaging of hemorrhagic PA was depended on the timeframe of the apoplectic duration [ 3]. In our study, we found that pituitary ring sign presented in 25.9% of ischemic PA patients, and 17.1% in patients with hemorrhagic PA. The pituitary ring sign can be found in both hemorrhagic PA and ischemic PA patients, and not all the hemorrhagic PA and ischemic PA patients present this sign, which is different to previous report. That means pituitary ring sign is not a predictor with high specificity for ischemic PA patients probably. Taken together, SSMT might be a diagnostic MRI feature of ischemic PA compared with hemorrhagic PA. The UK guidelines for the management of pituitary apoplexy recommended a score system to help us for making a strategic decision on surgical and conservative treatment of PA [ 14]. According to the guidelines, all the pituitary apoplexy cases enrolled in our study were received transsphenoidal pituitary operations in our center. The operative time and blood loss in operation were not statistically different between the ischemic and hemorrhagic PA patients. The incidence of postoperative cerebrospinal leakage and intracranial infection were similar. Rates of TTR and subsequent long-term outcomes were also similar in both of our study groups. Surgical intervention can improve neural functions of patients with pituitary apoplexy, but has limited influences on the recovery of hormones, except for thyroid function of ischemic PA patients. Up to now, there is not yet a coincident protocol the treatment of patients with pituitary apoplexy. Some reports, however, have also shown that conservative medical management can provide successful results. Chang et al. [ 4] reported that partial or complete visual recovery was identified in 90% of patients who underwent surgery and 94% of patients with conservative treatment. However, some authors have advocated urgent surgical procedures for better outcomes. Thus, it is still a hot topic for the treatment of patients with pituitary apoplexy. In our study, visual disturbances and cranial nervous functional disorder can be improved after urgent surgical intervention. We are inclined to believe that urgent surgical intervention for these patients with neuro-ophthalmic deficits should be reasonable. Our study is a retrospective and unicentral research, which has inherent limitations. Although we used every resource available, there are still some patients with missing data and lost follow-up, that limited the ability to generalize follow-up and outcomes data. Sixty-seven patients are included in our research with strict exclusion criteria, but this is still a limited sample study. And conservative treatment is considered to be efficient for some PA patients, but only patients with surgery are included, that may not be truly representative of the entire cohort. Although we separate ischemic from hemorrhagic PAs, they are not mutually exclusive and often both are seen in reality. According to previous reports, infarction in PAs showed large areas of coagulation necrosis with a little red blood clots or old blood in the histological specimen, but this still seem to be a little subjective. Thus, a larger, random, multicentric research is urgent for further study.
CONCLUSION
In conclusion, we found patients with ischemic PA presented different clinical characteristics to the hemorrhagic ones. Transsphenoidal surgery should be considered for ischemia or hemorrhagic PA patients with neuro-ophthalmic deficits and might benefit for pituitary thyroid axis recovery of ischemic PA patients.
Acknowledgements
This study was supported by Nursery Project of Army Medical University (No. 2019R054).
Fig. 1.
Representive magnetic resonance imaging (MRI) image and pathology of ischemic pituitary adenomas (PA). A-C : Show that typical MRI characteristics of ischemic PA (asterisk) in a coronal view : an iso- to hyperintense signal in a T1-weighted image (A), an iso- to hyperintense signal in a T2-weighted image (B), and uneven rim enhancement with internal hypointensity after gadolinium injection; mucosal thickening and enhancement of the sphenoid sinus (arrowhead) (C). D : Shows histopathological findings in PA with ischemia : large areas of pink, acellular, coagulative necrosis without normal tumor cell cytoarchitecture (Hematoxylin and Eosin stain, ×200). Bar, 60 μm. 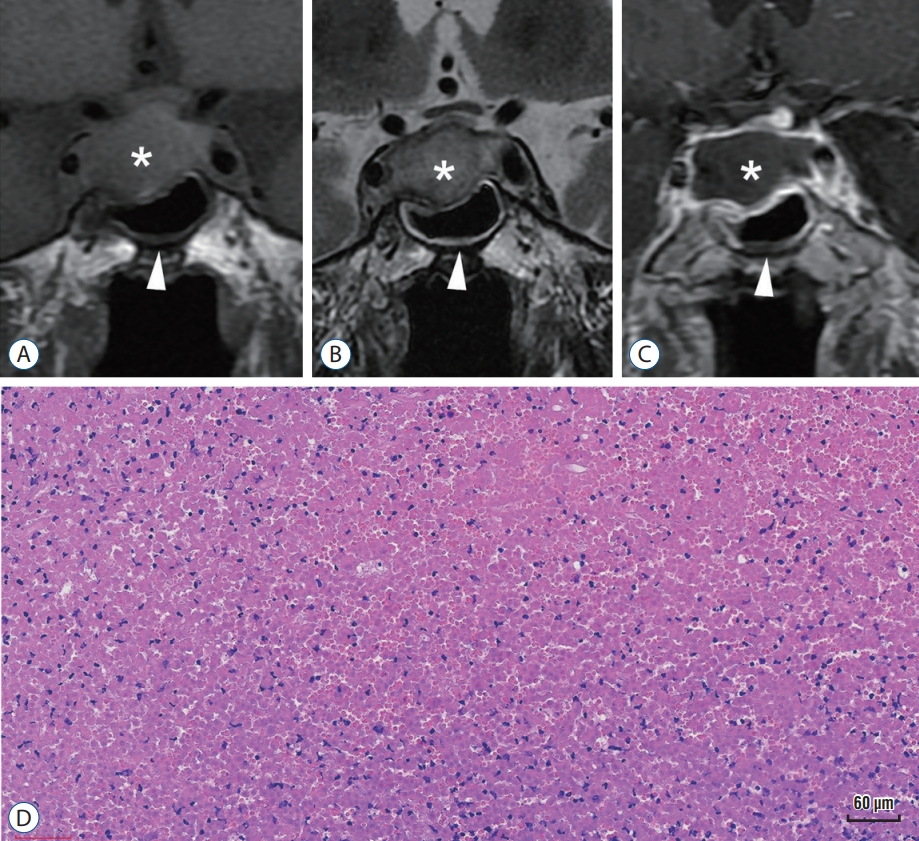
Fig. 2.
Representive magnetic resonance imaging (MRI) image and pathology of hemorrhagic pituitary adenomas (PA). A-C : Show that typical MRI characteristics of hemorrhagic PA (asterisk) in a coronal view : an iso- to hyperintense signal in a T1-weighted mass image, hemorrhagic components appear hyperintense signal in T1-weighted image (A), an iso- to hypointense signal in a T2-weighted mass image (B), and enhancement with internal hypointensity after gadolinium injection, but hemorrhagic components appear no enhancement (C). D : Shows histopathological findings in PA with hemorrhage : typical tumor cells surrounded by abundant red blood cells (Hematoxylin and Eosin stain, ×200). Bar, 60 μm. 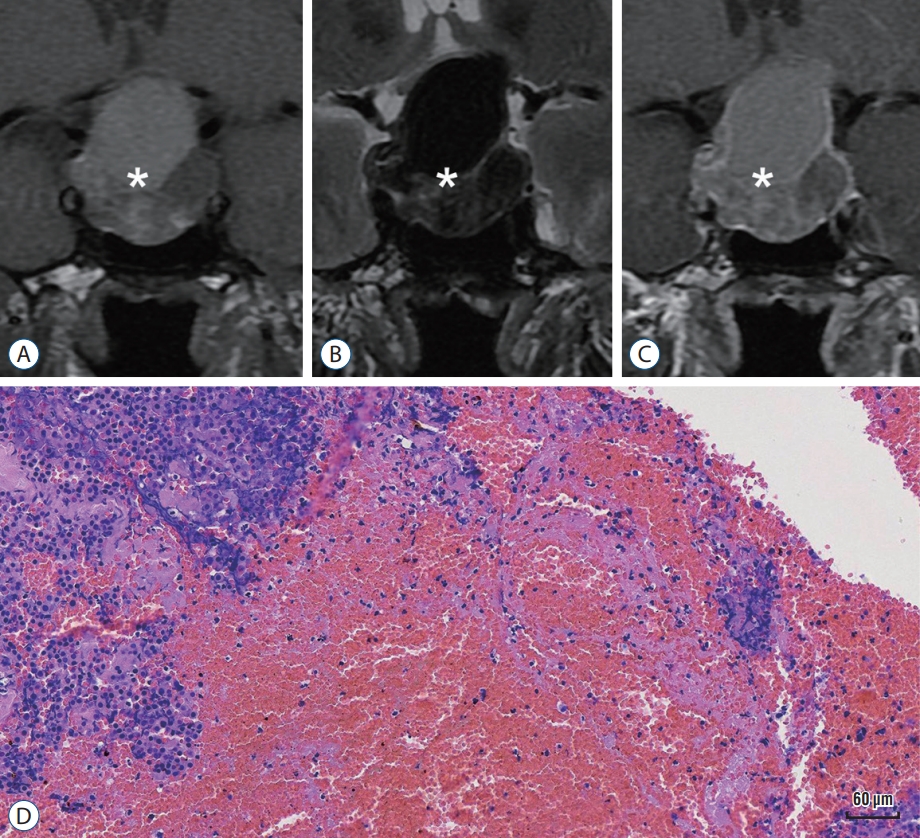
Table 1.
Baseline and clinical characteristics of 67 patients with apoplectic adenoma
|
Parameter |
Ischemia (n=28) |
Hemorrhage (n=39) |
p-value |
|
Sex |
|
|
0.043 |
|
Female |
6 |
18 |
|
|
Male |
22 |
21 |
|
|
Age (years) |
43.4±2.9 |
51.4±2.0 |
0.020 |
|
Mean tumor size (cm3) |
2.5×2.4×1.9 |
2.8×2.9×2.1 |
0.012 |
|
Tumor functionality (%) |
21.4 |
56.4 |
0.006 |
|
Preoperative hypopituitarism (%) |
60.7 |
48.7 |
0.457 |
|
Concomitant symptoms (%) |
|
|
|
|
Headache |
82.1 |
51.3 |
0.011 |
|
Neuroendocrine (%) |
|
|
|
|
Nausea/vomiting |
10.7 |
10.3 |
1.000 |
|
Weakness |
67.9 |
51.3 |
0.214 |
|
Hyponatremia |
51.8 (14/27) |
27.8 (10/36) |
0.069 |
|
Neurological (%) |
|
|
|
|
Vision loss or blurriness |
50.0 |
66.7 |
0.211 |
|
CN III, IV, and/or VI neuropathy |
42.9 |
56.4 |
0.319 |
|
MRI findings (%) |
|
|
|
|
Pituitary ring sign |
25.9 |
17.1 |
0.532 |
|
Sphenoid sinus mucosal thickening |
55.6 (15/27) |
0.0 (0/38) |
<0.0001 |
Table 2.
Characteristics of surgical treatment among apoplexy patients
|
Parameter |
Ischemia (n=28) |
Hemorrhage (n=39) |
p-value |
|
Operative time (minutes) |
69.3±6.9 |
82.3±8.0 |
0.246 |
|
Blood loss in operation (mL) |
97.3±16.9 |
192.9±62.5 |
0.208 |
|
Hospital stays |
15.0±1.3 |
14.6±0.9 |
0.751 |
|
Postoperative situation (%) |
|
|
|
|
Diabetes Insipidus |
46.4 |
48.7 |
1.000 |
|
Cerebrospinal leakage |
25.0 |
30.8 |
0.784 |
|
Intracranial infection |
7.14 |
7.69 |
1.000 |
|
Surgical outcomes |
|
|
|
|
Total tumor resection rate (%) |
94.1 |
92.9 |
1.000 |
|
Visual improvement |
13 (69.2) |
28 (85.7) |
0.237 |
|
Neuropathy improvement |
11 (81.8) |
19 (82.6) |
1.000 |
|
Drug use after discharge |
|
|
|
|
Hydrocortisone (mg) |
40.9±6.8 |
35.0±3.1 |
0.389 |
|
Levothyroxine (µg) |
33.0±4.6 |
27.6±3.7 |
0.352 |
Table 3.
Endocrine outcomes of patients with apoplectic adenomas
|
Parameter |
Ischemia
|
Hemorrhage
|
p-value*
|
|
Before |
After |
p-value |
Before |
After |
p-value |
|
Hypocortisolemia (%) |
46.4 |
37.0 |
0.588 |
35.9 |
23.3 |
0.301 |
0.453 |
|
hypothyroidism (%) |
46.4 |
18.5 |
0.044 |
28.2 |
19.4 |
0.418 |
0.196 |
|
hypogonadotropism (%) |
64.3 |
40.7 |
0.108 |
48.8 |
33.3 |
0.227 |
0.225 |
References
1. Agrawal B, Dziurzynski K, Salamat MS, Baskaya M : The temporal association of sphenoid sinus mucosal thickening on MR imaging with pituitary apoplexy. Turk Neurosurg 22 : 785-790, 2012   2. Arita K, Kurisu K, Tominaga A, Sugiyama K, Ikawa F, Yoshioka H, et al : Thickening of sphenoid sinus mucosa during the acute stage of pituitary apoplexy. J Neurosurg 95 : 897-901, 2001   4. Chang CV, Felicio AC, Toscanini AC, Teixeira MJ, Cunha-Neto MB : Pituitary tumor apoplexy. Arq Neuropsiquiatr 67 : 328-333, 2009   5. Falhammar H, Tornvall S, Höybye C : Pituitary apoplexy: a retrospective study of 33 cases from a single center. Front Endocrinol (Lausanne) 12 : 656950, 2021    6. Fernandez A, Karavitaki N, Wass JA : Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf) 72 : 377-382, 2010   7. Gupta P, Dutta P : Landscape of molecular events in pituitary apoplexy. Front Endocrinol (Lausanne) 9 : 107, 2018    8. Jho DH, Biller BM, Agarwalla PK, Swearingen B : Pituitary apoplexy: large surgical series with grading system. World Neurosurg 82 : 781-790, 2014   9. Liu JK, Couldwell WT : Pituitary apoplexy in the magnetic resonance imaging era: clinical significance of sphenoid sinus mucosal thickening. J Neurosurg 104 : 892-898, 2006   10. Ly S, Naman A, Chaufour-Higel B, Patey M, Arndt C, Delemer B, et al : Pituitary apoplexy and rivaroxaban. Pituitary 20 : 709-710, 2017    11. Ogawa Y, Niizuma K, Mugikura S, Tominaga T : Ischemic pituitary adenoma apoplexy-clinical appearance and prognosis after surgical intervention. Clin Neurol Neurosurg 148 : 142-146, 2016   12. Oldfield EH, Merrill MJ : Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg 122 : 1444-1449, 2015   13. Pyrgelis ES, Mavridis I, Meliou M : Presenting symptoms of pituitary apoplexy. J Neurol Surg A Cent Eur Neurosurg 79 : 52-59, 2018  14. Rajasekaran S, Vanderpump M, Baldeweg S, Drake W, Reddy N, Lanyon M, et al : UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (Oxf) 74 : 9-20, 2011   15. Vaphiades MS : Pituitary ring sign plus sphenoid sinus mucosal thickening: neuroimaging signs of pituitary apoplexy. Neuroophthalmology 41 : 306-309, 2017    16. Wang Z, Gao L, Wang W, Guo X, Feng C, Lian W, et al : Coagulative necrotic pituitary adenoma apoplexy: a retrospective study of 21 cases from a large pituitary center in China. Pituitary 22 : 13-28, 2019    18. Wildemberg LE, Glezer A, Bronstein MD, Gadelha MR : Apoplexy in nonfunctioning pituitary adenomas. Pituitary 21 : 138-144, 2018    19. Xiao D, Wang S, Huang Y, Zhao L, Wei L, Ding C : Clinical analysis of infarction in pituitary adenoma. Int J Clin Exp Med 8 : 7477-7486, 2015   20. Zhan R, Zhao Y, Wiebe TM, Li X : Acute hemorrhagic apoplectic pituitary adenoma: endoscopic management, surgical outcomes, and complications. J Craniofac Surg 26 : e510-515, 2015   21. Zhu Q, Liang Y, Fan Z, Liu Y, Zhou C, Zhang H, et al : Ischemic infarction of pituitary apoplexy: a retrospective study of 46 cases from a single tertiary center. Front Neurosci 15 : 808111, 2022   
|
|


















