Evran, Kayhan, Saygı, Ozbek, and Kilickesmez: Efficacy of Middle Meningeal Artery Embolization in Treatment Resistant Spontaneous Intracranial Hypotension Caused Subdural Hematoma : Report of Two Cases and Review of the Literature
Abstract
Spontaneous intracranial hypotension (SIH) most commonly manifests as bilateral subdural hematoma (SH). SIH cases mostly resolve spontaneously but further treatment would be needed via blind epidural blood patch (EBP). Cerebrospinal fluid (CSF) leakage in EBP-refractory cases can be treated surgically only if the localization of CSF leakage is detectable but it cannot be possible in most of the cases. Also surgical evacuation of SH secondary to SIH (SH-SIH) is not favorable without blocking the CSF leakage. Thus the management of these patients is a challenge and alternative treatment options are needed. Although middle meningeal artery embolization (MMAE) is an effective treatment option in non-SIH SH, there is no report about its application in the treatment of SH-SIH. We present two cases of SH-SIH which their clinical and radiological findings were completely resolved by bilateral MMAE treatment.
Key Words: Intracranial hypotension · Embolization · Meningeal arteries · Subdural hematoma.
INTRODUCTION
Spontaneous intracranial hypotension (SIH) is caused by leakage of cerebrospinal fluid (CSF) and a rare condition with an incidence of five people in 100.000 [ 8]. SIH is mostly presented as bilateral subdural hematoma (SH) radiologically [ 10]. The majority of SIH cases resolve spontaneously and the healing process is further improved with conservative treatments such as intravenous fluid, bed rest, oral salt, caffeine, theophylline, methylxanthine, and steroids. Epidural blood patch (EBP) can be applied when patients do not respond to conservative and pharmacological treatments, with a success rate of 70% to 90% [ 5, 18]. In failed cases despite multiple EBPs, surgical treatment is performed only if the localization of CSF leakage can be detected [ 12]. Also SH secondary to SIH (SH-SIH) cannot be evacuated with burr hole drainage sufficiently before the treatment of CSF leakage [ 17]. In this respect, the management of this patient group is challenging, and the alternative treatment modalities have come into question. Middle meningeal artery embolization (MMAE) is an effective treatment option in non-SIH subacute/chronic SH without surgical indication [ 13] however, there is no publication about treatment of SH-SIH with MMAE. Considering the surgical approach does not ensure the optimal treatment in EBP refractory SH-SIH, in this study we aimed to evaluate the effectiveness and applicability of MMAE in treatment resistant SH-SIH cases for the first time in literature.
CASE REPORT
Case 1
A 40-years-old male presented with orthostatic headache and bilaterally subdural hematoma was detected in his cranial computed tomography (CT)-scan with a volume of 24.23, 12.25, and 11.98 mL in total, left side and right side respectively. Subdural hematoma volumes were evaluated by OsiriX ® Dicom Viewer program (Pixmeo SARL, Bernex, Switzerland) and calculated with Manuel Planimetric Method. While dural enhancement was detected in cranial magnetic resonance imaging (MRI) scans, there were no pathology causes to CSF leakage in whole-spinal MRI. The patient was diagnosed as SIH according to International Classification of Headache Disorders criteria [ 11] after the assessments include anamnesis, examination and MRI scans ( Fig. 1). The patient got conservative treatment, which includes bed rest at 20-30° Trendelenburg position, hydration, intravenous steroid, oral salt and caffeine. After the 7 days follow-up with conservative treatment, patient did not show any clinical improvement. Then blind EBP was performed with 5-10 mL of 1% lidocaine in a monoplane digital subtraction angiography (DSA) suite (Siemens Artis Zee, Erlangen, Germany) in the left lateral decubitus position, with autologous blood-contrast medium mixture (5 mL of iopamidol). Thirty mL venous blood sample was obtained from antecubital vein and it was administered into the lumbar epidural region with 20-gauge spinal needle. After EBP, patient was observed for 7 days and his medical treatments were continued with resting in the Trendelenburg position. The second session of EBP was performed to the patient because of he did not improve within 7 days after the first session. Conservative treatment procedures were sustained to the patient who got second session of EBP, as done after the first session. Control CT scans were performed at the 6th day of conservative, first EBP and second EBP treatment steps and no regression in hematoma volumes was noted.
Bilateral MMAE was performed to the patient who did not exhibit any clinical improvement despite 21 days of conservative treatment and two sessions of EBP with 1 week intervals. MMAE was performed under conscious sedation in a monoplane DSA suite. Access was achieved from the right femoral artery with 5 F introducer; following 5000 IU of IV heparin administration, both external carotid arteries were catheterized with 5 F diagnostic catheters, and middle meningeal artery (MMA) catheterization was performed coaxially with 2.7 F Progreat ® microcatheter (Terumo, Tokyo, Japan). One vial of 100-300 micron Bead Block ® (Bead Block; Biocompatibles, Farnham, England) was used until stasis occurred ( Fig. 2). Conservative treatment procedures were carried on to the patient after MMAE, as done after EBP sessions. After MMAE, patient was followed-up for 1 week. At the end of first week SH volumes were minimally decreased at CT scan and patient was partially relieved compared to first of onset. Patient was discharged 1 week after MMAE. Control cranial CT and MRI scans were performed at the end of 1st and 3rd months following the MMAE. Complete resolution of SH was observed in imagings at the end of 3rd month and patient did not have any complaints ( Fig. 3).
Case 2
A 39-years-old male patient presented with dizziness and his cranial CT-scan demonstrated bilaterally subdural hematoma with a volume of 25.83, 12.65, and 13.18 mL at total, left side, and right side, respectively ( Fig. 4). Dural enhancement was seen in his contrasted cranial MRI but no pathology causes to CSF leakage were detected in whole spinal MRI and MRI myelography. The patient was diagnosed as SIH and conservative treatments were applied for 7 days. After 7 days, blind EBP was performed since conservative treatment did not provide any relief in complaints and regression of hematoma volume. Then the second session of blind EBP was performed at the 14th day because of the patient did not show any improvement clinically and radiologically. During these periods, conservative treatment was continued without interruption and control CT scans were done at the 6th day of conservative, first and second EBP sessions. No regression of hematoma volumes and complaints of patient were observed at the end of 21 days despite conservative and two sessions of EBP treatments.
Bilateral MMAE was performed to the patient at the 21st day of his intervention due to persistent clinical and radiological findings. After the procedure, conservative treatment was maintained. One week after MMAE, minimal decrease in hematoma volume was observed in the CT scan and the complaints were partially reduced. Patient was discharged at first week of the procedure and patient was followed up with cranial CT and MRI scans at the end of 1st and 3rd months of MMAE. At the 3rd month control patient had complete resolution of hematoma in radiological imagings and not any complaints ( Fig. 5).
DISCUSSION
The most common natural course of SIH is spontaneous resolution and it occurs within several weeks. Conservative treatment options may support this spontaneous resolution process. Autologous EBP may be performed in patients who do not improve with conservative treatment and has 70-90% success rate [ 5, 18]. Although superiority of myelography-guided targeted EBP to blind EBP was reported [ 1], Cho et al. [ 3] indicated that these two techniques has similar results. Regarding the current literature for EBP treatment in SIH patients, Wu et al. [ 18] reported the 98% complete relief after first two EBP sessions which were performed in 2 days intervals; and Chung et al. [ 5] published the majority of the cases were relieved within 1 month after EBP. In our current issue, we performed MMAE after almost 1 month follow-up and noted promising results in this SIH with bilateral SH patients which have severe symptoms and radiological herniation findings despite two sessions of EBP and conservative treatment. Considering the management of these patient group is challenging and controversial, we concluded that the MMAE can be a beneficial option for SH-SIH patients whose symptoms did not relieve with EBP treatment. Drainage of collection and irrigation of subdural space with burr hole, twist-drill craniostomy and craniotomy are frequently used surgical techniques in non-SIH subacute/chronic SH [ 19]. However SH-SIH often progresses into chronic SH [ 19]; but evacuating the hematoma before treatment of the CSF leakage may cause hematoma recurrence and even clinical worsening. García-Morales et al. [ 9] reported recurrence after 2 months of hematoma drainage in a patient with SH-SIH. In a series of 40 patients, Schievink et al. [ 16] reported that the hematoma did not regress without treatment of CSF leakage in which hematoma had been evacuated prior to the treatment of CSF leakage. The authors further advocated that hematoma drainage is unnecessary and CSF leakage can be treated safely before evacuating the hematoma [ 16]. Dhillon et al. [ 6] reported a SIH case that worsened clinically whose hematoma was evacuated initially; therefore, they also recommended repair of CSF leakage as first-step treatment. Other studies in the literature also reported that it is unnecessary to evacuate the hematoma first, which might cause clinical deterioration [ 4, 7]. Although repair of the CSF leakage before surgical SH drainage is an appropriate treatment option for SH-SIH patients, there is no well accepted intervention for SH-SIH cases with no detected CSF leakage origin like our patients. In this respect, MMAE may be an option to close the gap in this area. In non-SIH subacute/chronic SH without surgical indication, MMAE is an effective treatment option. The effectiveness of embolization in both newly diagnosed and recurrent hematoma was often reported in the literature [ 13- 15]. The underlying mechanism of regression of the hematoma with embolization is the disruption of the feeding of the outer membrane of the hematoma originating from the dura. This membrane seems to help the evolution of hematoma, which was demonstrated with several studies radiologically and histologically [ 2, 13]. In this study, SH-SIH did not regress initially despite conservative and medical treatment and further two sessions of EBP. Therefore, MMAE, as a minimally invasive treatment method, was performed for the first time in the literature in SH-SIH. We observed that the hematoma was completely resorbed, as documented radiologically after 3 months. With this study, we present to the literature that MMAE may be an alternative treatment option in EBP-refractory SH-SIH patients who are not suitable for surgery. Considering the small number of cases in our study, we think that our findings should be supported with studies including more cases, but we believe that our findings will inspire future studies.
CONCLUSION
The diagnosis and treatment of SIH may be challenging. Conservative methods and EBP are the 1st choice for treatment but EBP has a success rate of 70-90%. EBP-refractory cases are surgically treated only if the localization of CSF leakage can be detected. At this point, it was observed that there was an inadequacy in the treatment of a patient group which is resistant to EBP and not appropriate for surgery. Our results showed that MMAE may be an effective treatment method for EBP-reftactory SH cases of SIH. Our study will lead further studies with larger patient groups to investigate this challenging issue.
Fig. 1.
Preoperative magnetic resonance imaging (MRI) of epidural blood patch (ebP)-refractory case 1. A : Axial T2-weighted MRI shows bilateral widening of subdural spaces and subdural hematoma (SH) (arrows). b : Axial T2-weighted MRI at the level of the uncus shows obliteration of bilateral sylvian and basal cisterns (red arrows) and bilateral uncal herniation especially on the left side (blue arrow). c : coronal fluid-attenuated inversion recovery MRI shows bilateral SH (red arrows), shrinkage of lateral ventricles (blue arrows), obliteration of the third ventricle and bilateral uncal herniation especially on the left side (yellow arrow). d : Sagittal T1-weighted MRI shows shrinkage of corpus callosum (red arrows), reduction of pontomesencephalic angle, mesencephalic compression due to transtentorial herniation, and tonsillar herniation (blue arrow). e : Sagittal enhanced T1- weighted MRI shows epidural venous congestion extending to c4 level (red arrows). f : Axial enhanced T1-weighted MRI shows increase of pachymeningeal enhancement (red arrows) and bilateral SH (blue arrows). 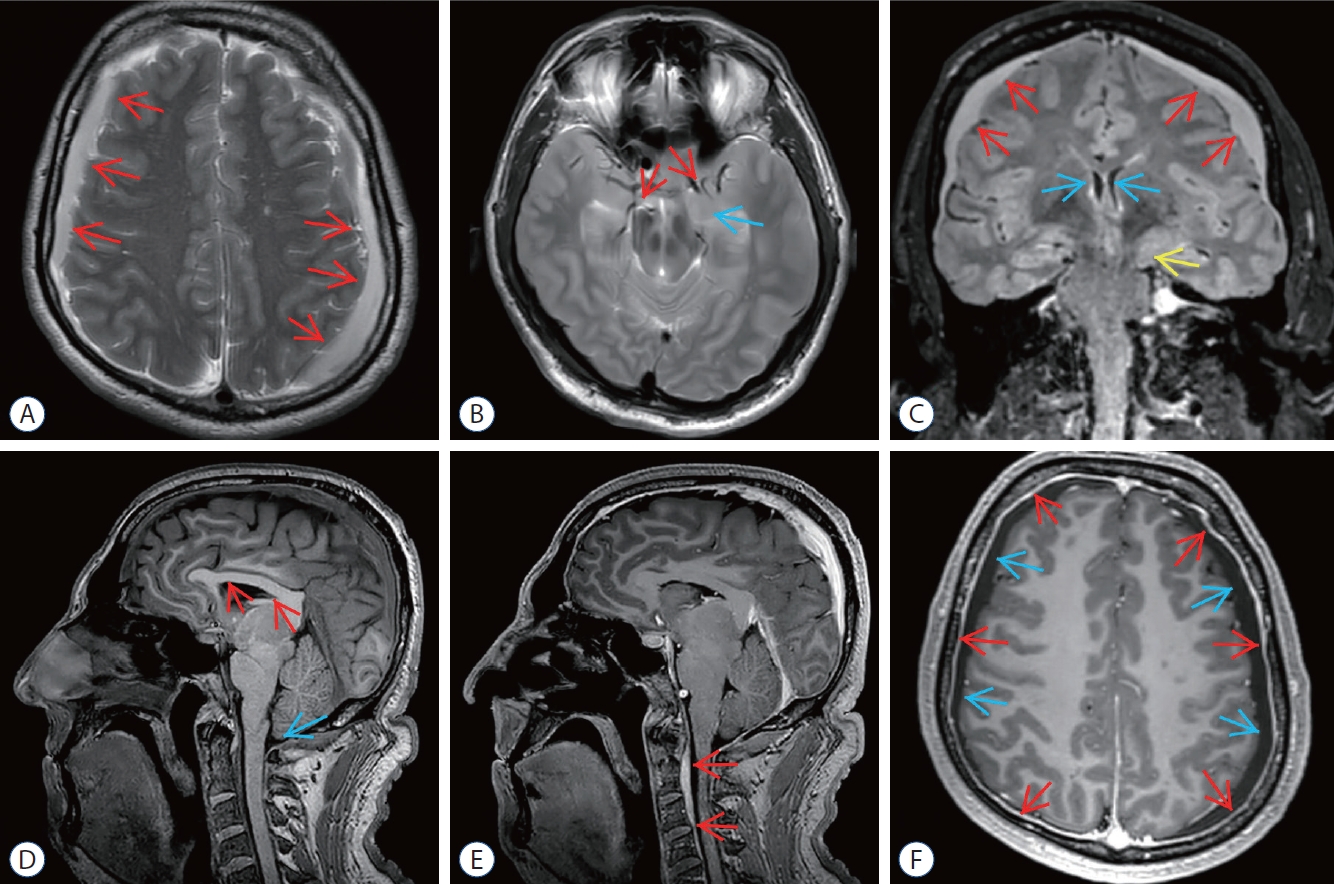
Fig. 2.
Angiographic images of middle meningeal artery embolization (MMAe). A : Pre embolization lateral image of the right middle meningeal artery (MMA) (arrow). b : Post embolization lateral image shows end-branch pruning of the right MMA (arrows). c : Pre embolization lateral image of the left MMA (arrow). d : Post embolization lateral image shows end-branch pruning of the left MMA (arrows). 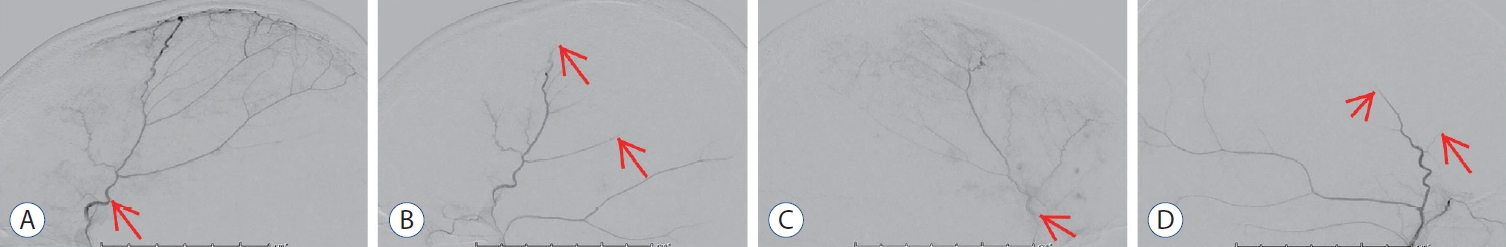
Fig. 3.
Post embolization magnetic resonance imaging (MRI) of epidural blood patch (ebP)-refractory case 1. A : Axial T2-weighted MRI at the level of centrum semiovale shows total regression of subdural hematoma (SH) and better sulcus-gyrus differentiation (arrows). b : Axial T2-weighted MRI at the level of the third ventricle shows enlarging of the third ventricle and bilateral sylvian cisterns (arrows). c : coronal T2-weighted MRI shows enlarging of the lateral and third ventricles (blue arrows), total regression of SH and improving of bilateral uncal herniation (red arrows). d : Sagittal T2-weighted MRI shows regression of the shrinkage of corpus callosum and increment of the pontomesencephalic angle (arrows). 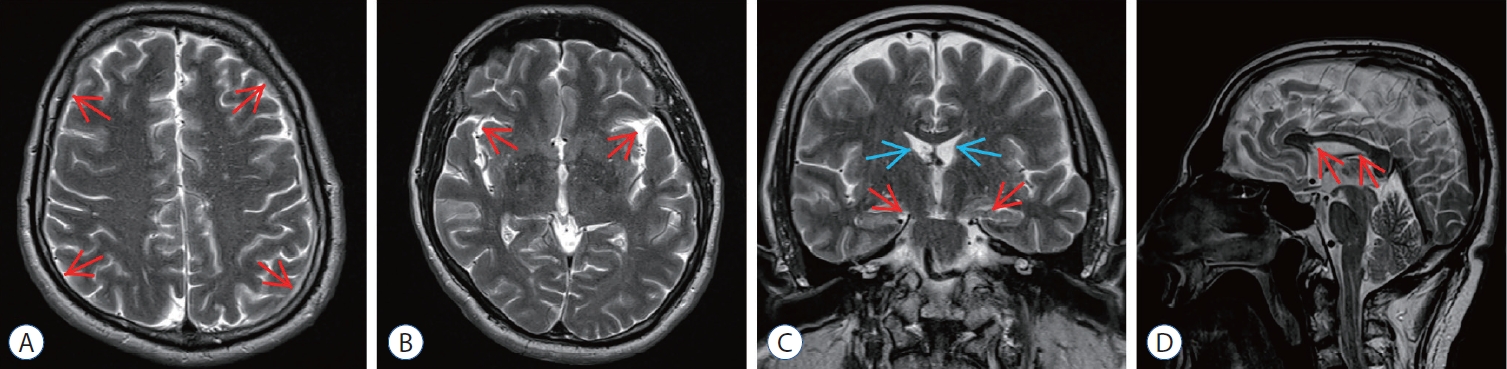
Fig. 4.
Preoperative computed tomography (cT) scans of epidural blood patch (ebP)-refractory case 2. A : Axial cT image at the level of centrum semiovale shows bilateral subdural hematoma (SH) and difficulty of sulcus-gyrus differentiation (arrows). b : Axial cT image at the level of basal cisterns shows obliteration in sylvian and basal cisterns (red arrows) and third ventricle (blue arrow). c : coronal cT image shows bilateral SH (red arrows), shrinkage of lateral ventricles (blue arrows), and obliteration of the third ventricle (yellow arrow). 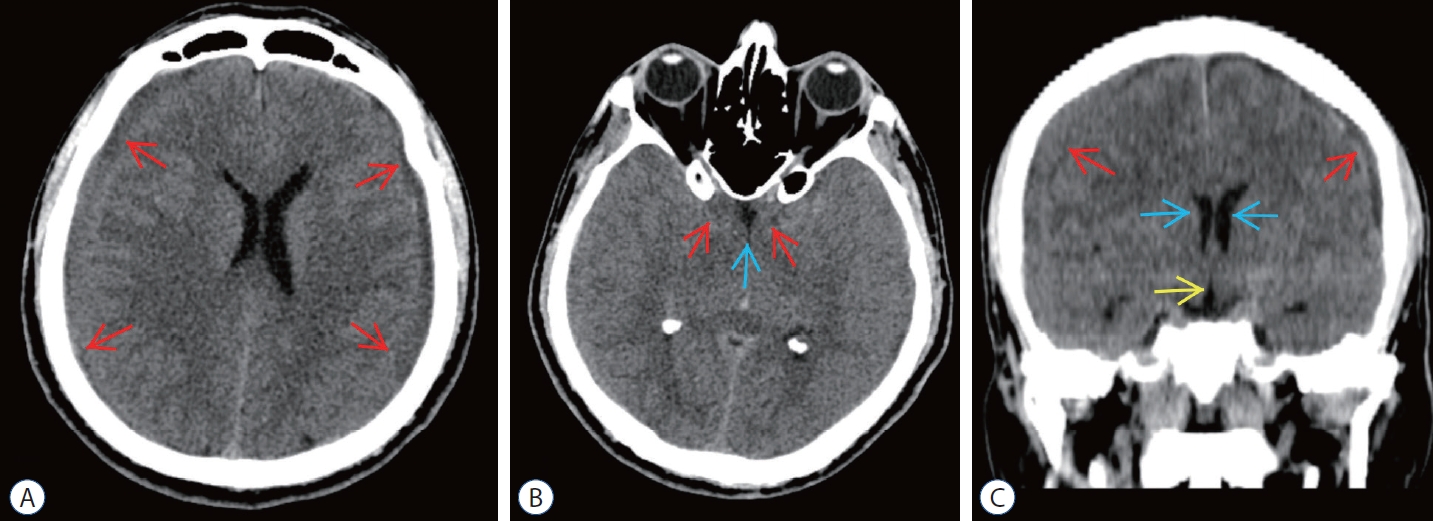
Fig. 5.
Post embolization computed tomography (cT) scans of epidural blood patch (ebP)-refractory case 2. A : Axial cT image at the level of centrum semiovale shows total regression of the subdural hematoma and better sulcus-gyrus differentiation (arrows). b : Axial cT image at the level of the third ventricle shows enlarging of the third ventricle (blue arrow) and bilateral sylvian cisterns (red arrows). c : coronal cT image shows enlarging of the lateral and third ventricles (blue arrows) and bilateral sylvian cisterns (red arrows) and also total regression of SH. 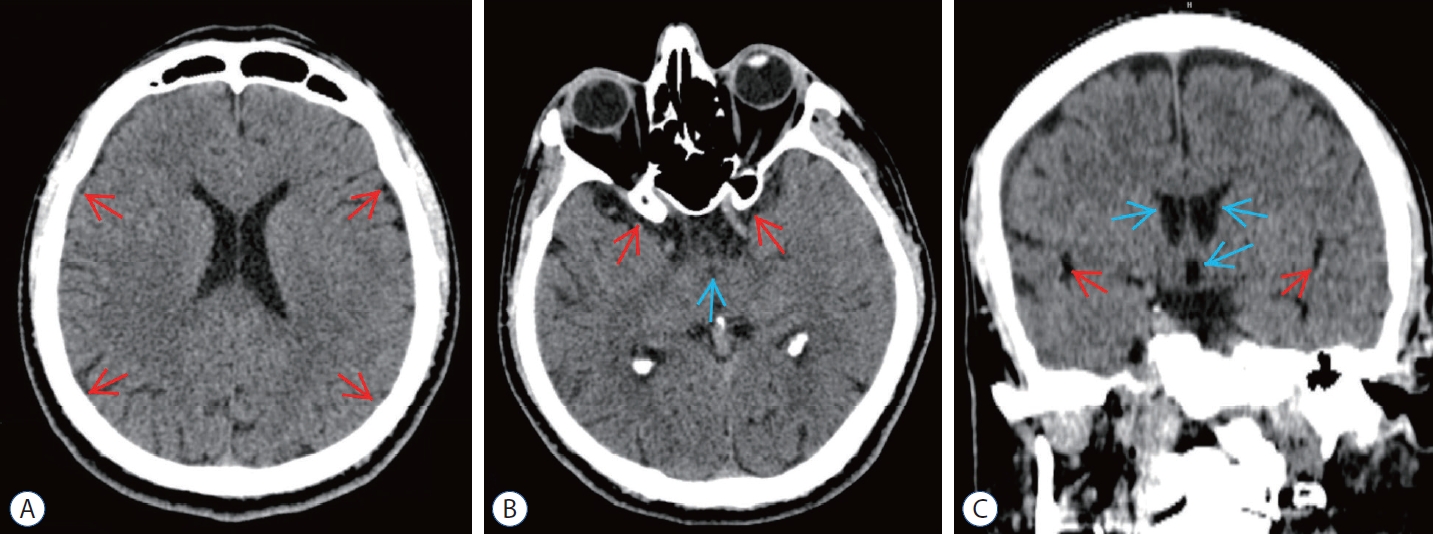
References
1. Ahn C, Lee E, Lee JW, Chee CG, Kang Y, Kang HS : Two-site blind epidural blood patch versus targeted epidural blood patch in spontaneous intracranial hypotension. J Clin Neurosci 62 : 147-154, 2019   2. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al : Middle meningeal artery embolization for chronic subdural hematoma. Radiology 286 : 992-999, 2018   3. Cho KI, Moon HS, Jeon HJ, Park K, Kong DS : Spontaneous intracranial hypotension: efficacy of radiologic targeting vs blind blood patch. Neurology 76 : 1139-1144, 2011   4. Chotai S, Kim JH, Kim JH, Kwon TH : Brain herniation induced by drainage of subdural hematoma in spontaneous intracranial hypotension. Asian J Neurosurg 8 : 112-115, 2013    5. Chung SJ, Kim JS, Lee MC : Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology 55 : 1321-1327, 2000   6. Dhillon AK, Rabinstein AA, Wijdicks EF : Coma from worsening spontaneous intracranial hypotension after subdural hematoma evacuation. Neurocrit Care 12 : 390-394, 2010    7. Ferrante E, Rubino F, Beretta F, Regna-Gladin C, Ferrante MM : Treatment and outcome of subdural hematoma in patients with spontaneous intracranial hypotension: a report of 35 cases. Acta Neurol Belg 118 : 61-70, 2018    8. Forghani R, Farb RI : Diagnosis and temporal evolution of signs of intracranial hypotension on MRI of the brain. Neuroradiology 50 : 1025-1034, 2008    9. García-Morales I, Porta-Etessam J, Galán L, Lagares A, Molina JA : Recurrent subdural haematomas in a patient with spontaneous intracranial hypotension. Cephalalgia 21 : 703-705, 2001    10. Girão MMV, Sousa RMP, Ribeiro MC, Cardoso TAM de O, França Júnior MC, Reis F : Spontaneous intracranial hypotension and its complications. Arq Neuropsiquiatr 76 : 507-511, 2018   11. Headache Classification Committee of the International Headache Society (IHS) : The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33 : 629-808, 2013    12. Inenaga C, Tanaka T, Sakai N, Nishizawa S : Diagnostic and surgical strategies for intractable spontaneous intracranial hypotension. Case report. J Neurosurg 94 : 642-645, 2001  13. Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J : Middle meningeal artery embolization as treatment for chronic subdural hematoma: a case series. Oper Neurosurg (Hagerstown) 14 : 556-562, 2018    14. Link TW, Boddu S, Paine SM, Kamel H, Knopman J : Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery 85 : 801-807, 2019    15. Link TW, Schwarz JT, Paine SM, Kamel H, Knopman J : Middle meningeal artery embolization for recurrent chronic subdural hematoma: a case series. World Neurosurg 118 : e570-e574, 2018   16. Schievink WI, Maya MM, Moser FG, Tourje J : Spectrum of subdural fluid collections in spontaneous intracranial hypotension. J Neurosurg 103 : 608-613, 2005   17. Takahashi K, Mima T, Akiba Y : Chronic subdural hematoma associated with spontaneous intracranial hypotension: therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo) 56 : 69-76, 2016    18. Wu JW, Hseu SS, Fuh JL, Lirng JF, Wang YF, Chen WT, et al : Factors predicting response to the first epidural blood patch in spontaneous intracranial hypotension. Brain 140 : 344-352, 2017   19. Yadav YR, Parihar V, Namdev H, Bajaj J : Chronic subdural hematoma. Asian J Neurosurg 11 : 330-342, 2016   
|
|





















