Park, Jung, Park, and Kwon: Could A1 Aplasia or Hypoplasia Affect the Morphology and Rupture Risk of Anterior Communicating Artery Aneurysm?
Abstract
Objective
Anterior communicating artery (Acom) aneurysm is one of the most common intracranial aneurysms, constituting approximately 30-35% of all aneurysm formation in the brain. Anatomically, the H-complex (the anatomic morphology of both A1 to A2 segments) is thought to affects the nature of the Acom aneurysm due to its close relationship with the hemodynamics of the vessel. Therefore, we investigated the relative risk factors of aneurysmal rupture, especially focusing on H-complex morphology of the Acom.
Methods
From January 2016 to December 2020, a total of 209 patients who underwent surgery, including clipping and coiling for Acom aneurysm in our institution were reviewed. There were 102 cases of ruptured aneurysm and 107 cases of unruptured aneurysm. The baseline morphology of aneurysms was investigated and the relationship between the H-complex and the clinical characteristics of patients with Acom aneurysms was assessed.
Results
Of the 209 patients, 109 patients (52.1%) had symmetrical A1, 79 patients (37.8%) had unilateral hypoplastic A1, and 21 patients (10.0%) had aplastic A1. The hypoplastic A1 group and the aplastic A1 group were grouped together as unilateral dominancy of A1, and were compared with the symmetrical A1 group. There was no significant difference in demographic characteristics and radiological findings of Acom aneurysms between two groups. However, when dichotomizing the patients into ruptured cases and unruptured cases, unilateral dominance of the A1 segment was associated with aneurysmal rupture with statistical significance (p=0.011).
Conclusion
These results suggest that the unilateral dominance of the A1 segment does not have a significant effect on the morphology of Acom aneurysms, but contributes to aneurysmal rupture. Thus, we can better understand the effects of hemodynamics on Acom aneurysm.
Key Words: Anterior cerebral artery · Intracranial aneurysm · Rupture · Subarachnoid hemorrhage · Hypoplasia.
INTRODUCTION
The general incidence of intracranial aneurysm is reported to be about 2-5% [ 20]. However, only 1% of all intracranial aneurysm actually rupture [ 24]. A ruptured aneurysm causes subarachnoid hemorrhage (SAH) that causes 12% of sudden deaths on postmortem studies and has a 30-day mortality rate as high as 45% [ 3]. Anterior communicating artery (Acom) aneurysm is one of the most common intracranial aneurysms, constituting approximately 30-35% of all aneurysm formations in the brain [ 1, 8]. It has the greatest rupture risk compared to aneurysms in any other locations [ 7, 15]. Considering its prevalence and risk of bleeding, predicting the relative rupture risk of unruptured Acom aneurysms has a great value in preventing a catastrophic event related to SAH, and its associated high mortality and morbidity rates [ 11]. Multiple external factors act together with predisposing genetic background, contributing to the formation of intracranial aneurysms [ 2]. In particular, there have been increasing interests in the effect of the blood flow and its hemodynamics on the pathogenesis of cerebral aneurysms. The H-complex, which classifies the anatomical morphology of the Acom according to the relationship between the A1 and A2 segments, is considered to affect the hemodynamics of the Acom [ 12]. When one A1 dominantly supplies both anterior cerebral arteries (ACA), it is thought to have a causal relationship with the development of Acom aneurysms due to increased pressure to the vessel wall by the termination of blood flow [ 13, 22]. In a previous study, Rinaldo et al. [ 18] reported that the presence of hypoplasia or aplasia of the A1 segment were associated with Acom aneurysm formation due to hemodynamic stress from unbalanced blood flow in the Acom. Moreover, the dominance of the A1 segment has been reported to be a significant risk factor for predicting the rupture of Acom aneurysms, subsequently leading to SAH or parenchymal hemorrhage [ 19]. However, the exact relationship between the anatomical variants of the H-complex and the rate of aneurysm formation or aneurysm rupture is not well understood [ 13]. Herein, we conducted this retrospective study to find out whether differences in rupture rates exist in hypoplasia/aplasia A1 compared to A1 with symmetrical vessel morphology. In addition, we also analyzed the various risk factors of Acom aneurysm rupture (SAH) based on demographic characteristics and radiological findings of the Acom [ 6].
MATERIALS AND METHODS
Patient population and inclusion criteria
The Local Institutional Review Board of Ulsan University Hospital (Ulsan, Korea) approved this retrospective study (IRB No. 2021-07-051). This is retrospective study through electronic chart review. We included adult patients (>18 years old) who underwent surgery (clipping or endovascular coiling) for their Acom aneurysm (both ruptured and unruptured) from January 2016 to December 2020 in our institution (a tertiary medical center). Exclusion criteria included; 1) cases with chronic internal carotid artery (ICA) occlusion, 2) intraoperatively ruptured aneurysm, and 3) cases without digital subtraction angiography (DSA). In other words, all cases where the sizes of both the A1 artery and aneurysm could be measured through DSA images for patients treated for aneurysm during this period were included.
Data measurements and determination of vessel type
The morphology of aneurysm was analyzed based on the DSA findings. The included patients were divided into three groups by morphological features of the H-complex : symmetric A1, unilateral hypoplastic A1, and unilateral aplastic A1 ( Fig 1). The ‘hypoplastic’ A1 segment was determined as the ratio of vessel width, which meant that the smaller A1 segment was measured to be less than 50% of the contralateral, larger A1 segment. Aplasia means the absence of one side of the A1 segment. Then, we classified the patients into two groups according to the symmetry of the A1 (symmetric A1 group and asymmetric A1 group). Asymmetric groups included both cases with unilateral hypoplastic and aplastic A1. Other angiographic measurements comprised the maximal diameter of the aneurysmal sac, the presence of daughter sacs, the dome-to-neck ratio (defined as the dome width of aneurysm divided by the neck width), and the aspect ratio (defined as the perpendicular height of the aneurysm divided by the neck with) to analyze the risk factors of aneurysm rupture ( Fig. 2).
Covariates and data analysis
The first consideration of this study was to compare the demographic and radiological differences between the symmetric A1 group and the asymmetric A1 group to analyze the factors related to aneurysm morphology. Demographic covariates included age, sex, smoking history (current or past), history of hypertension (HTN) and diabetes mellitus (DM), and body mass index (BMI). Radiological comparison points were the maximal diameter of the aneurysmal sac, dome-to-neck ratio, aspect ratio, and the presence of daughter sacs. Then, we divided the patients again into ruptured and unruptured groups to compare the risk factors of SAH. The risk factors of SAH included demographic and radiological characteristics as described above. The presence of asymmetric A1 was also compared to see whether or not it contributed to aneurysmal rupture.
Statistical analysis
All statistical analyses were performed using SPSS version 23 (IBM Corporation, Chicago, IL, USA). Categorical variables were reported as frequencies and percentages, and were compared using the chi square test. Continuous variables were analyzed for between-group comparison using the two-sample Student’s t-test. Then, multivariate logistic regression analysis was done for the variables which has statistical significance in the univariate study. In this analysis, relative rupture risk was assessed by odds ratios (ORs) with 95% confidence intervals (CIs). All statistical tests have the alpha level set at 0.05 for statistical significance.
RESULTS
Overall, 231 patients underwent surgical treatments (clipping or coiling) for Acom aneurysms in our center from January 2016 to December 2020. Twenty-two cases were excluded due to cases of chronic ICA occlusion, intra-op ruptures, or cases that did not undergo DSA. Consequently, a total of 209 cases were included, involving 102 cases (48.8%) of ruptured aneurysms (SAH) and 107 cases (51.2%) of unruptured aneurysms. A total of 12 patients underwent aneurysmal clipping surgery and 197 patients underwent endovascular treatment.
According to the morphology of the H-complex, there were a total of three predominant types, including a group with symmetrical A1, a group showing aplasia of the A1 on one side, and a group with hypoplasia of the A1 on one side. Then, we divided the patients into two groups again according to the symmetrical features of the A1. The detailed comparison of demographic and radiological differences of the two groups as divided by the presence of symmetrical A1 is described in Table 1. One-hundred-and-nine of the 209 cases (52.2%) have symmetrical A1 and 100 of the 209 (47.8%) cases have asymmetrical A1 (hypoplasia or aplasia). There were no differences in the patient characteristics and morphology of aneurysms between the two groups. Although the size of the aneurysms and the aspect ratio are thought to be related to unilateral dominance of the A1 segment, there was no statistical significance. Then, in the cases with unilateral A1 dominancy, the frequency of right A1 hypoplasia or aplasia, and left A1 hypoplasia or aplasia was 82.7% and 17.3% in the ruptured group and 83.3% and 16.7% in the unruptured group, respectively. Although the ratio of hypoplasia or aplasia of the right A1 was high in each group, there was no significant difference in the ratio of hypoplasia or aplasia of the right A1 in each of the two groups.
Then, the patients were divided into the ruptured group and the unruptured group again; the mean age of the two groups was 56.62±11.95 and 62.84±9.42 years, respectively ( Table 2). The risk factors of SAH, including smoking history, current smoking status, history of HTN and DM, morphology of aneurysm, presence of daughter sacs, and BMI was calculated ( Table 2). The presence of unilateral dominance of the A1 segment was also compared. Comparing the unruptured and ruptured groups, relatively younger age (median, 62.84±9.42 vs. 56.62±11.95; p<0.01), current smoking status (23.4% vs. 44.1%, p<0.01), aneurysm size (median, 4.81±2.22 vs. 6.10±2.54; p<0.01), the presence of daughter sacs (41.1% vs. 71.6%, p<0.01), and larger aspect ratio (median, 1.40±0.85 vs. 1.62±0.62; p=0.041) were significantly related to SAH in the univariate analysis. However, BMI (median, 24.92±3.32 vs. 23.69±3.39; p<0.009) was higher in the unruptured group. The presence of hypoplastic or aplastic A1 also increased the risk of aneurysmal rupture (39.3% vs. 56.9%, p=0.011). In the multivariate study, each covariate which achieved statistical significance in the univariate analysis were demonstrated in Table 3. Age (OR, 0.946; 95% CI, 0.914-0.979; p<0.01) and BMI (OR, 0.909; 95% CI, 0.827-0.999; p=0.047) were negatively correlated with SAH occurrence. Meanwhile, aneurysm size (OR, 1.193; 95% CI, 1.023-1.390; p=0.024), presence of daughter sacs (OR, 2.911; 95% CI, 1.503-5.638; p<0.01), and dominance of unilateral A1 segment (OR, 2.007; 95% CI, 1.055-3.820; p=0.034) positively increased the risk of aneurysmal rupture.
DISCUSSION
A1 segment hypoplasia or aplasia are common anatomical variants which are found in patients who have Acom aneurysms [ 10]. In other studies, Acom aneurysms found more frequently with A1 segment hypoplasia. Moreover, for the Acom aneurysms with A1 segment hypoplasia, they have larger and greater dome-to-neck ratios [ 18, 22]. In cadaveric studies, disproportionately more Acom aneurysms were found with unilateral dominancy on A1 segments [ 16]. So, we investigated the patient characteristics to uncover what extent A1 hypoplasia may affect the formation of Acom aneurysm. However, in this study, no significant association was found. This result is thought to have occurred due to the small number of patients enrolled. In order to properly evaluate this, a study involving more patients will be needed. In addition, in Table 1, we investigated whether there was a difference in the characteristics of the patient groups for the presence or absence of A1 hypoplasia. Age, HTN, DM, smoking history, and other patient-related factors did not significantly contribute to the occurrence of A1 hypoplasia. Considering this, it is thought that A1 hypoplasia can be used as an independent factor to evaluate how much it affects the rupture of aneurysms. Previous studies revealed that old age, female sex, HTN, DM, smoking history, presence of daughter sacs, size of aneurysm, and aspect ratio are risk factors that can affect aneurysm rupture [ 5, 9, 21]. We tried to analyze whether the well-known risk factors of SAH actually affect the occurrence of aneurysmal rupture and to find out other undetermined factors that contribute to aneurysm rupture. As expected, larger aneurysm size and the presence of daughter sacs significantly increased the risk of the incidence of SAH. However, HTN showed no significant correlation with aneurysm rupture in this study. Some reasons can be considered for this result. First, the patients in unruptured group tend to have regular health check-ups, so the prevalence of HTN in unruptured group could be overestimated. Second, poor history taking for the patients with poor mental status in the ruptured group could affect the statistical results. Although it is generally known that SAH occurs more frequently in older patients [ 21], the opposite result was found in this study. This difference may be explained by the fact that health check-ups are mainly performed for older people in this study, so preventive treatment was performed more in older patients than younger ones. In addition, there was a tendency of more brittle aneurysms found in relatively younger patients in our SAH group. We also found that BMI tended to be lower in the ruptured group than in the unruptured group. In one study, increased BMI can be associated with lower aneurysm rupture risk [ 4]. However, in this study, in terms of BMI, the median value of BMI in this study was within the normal range (24.9 vs. 23.7), and neither groups were obese. We thought that a patient with a ruptured aneurysm would have had poor oral intake before coming to the hospital, consequently leading to dehydration and lower body weight. Previous studies showed that increased flow in the ACA due to unilateral hypoplastic A1 is correlated to shear stress to the vessel wall, which contributes to the hemodynamic change in the ACA [ 12, 23]. These findings are consistent with the findings that A1 hypoplasia may affect the morphology of aneurysm [ 19]. However, in our study, hypoplasia or aplasia of A1 did not have a statistically significant effect on the morphology of aneurysm. Instead, in our study, hypoplastic A1 was found to affect the rupture risk of aneurysm. For the reason, we thought about the nature of vessels surrounding the aneurysm. When the flow on one side of the A1 is anatomically restricted, the hemodynamics of the Acom aneurysm mimics termination type aneurysm, and the termination type aneurysm ruptures more easily considering the hemodynamics within the aneurysm [ 17, 22]. Given the potential effects on arterial wall shear stress, it is possible that A1 segment hypoplasia may also confer an increased risk of Acom aneurysm rupture. In addition, we discovered one interesting point; hypoplasia or aplasia was more commonly observed on the right side than on the left (83% vs. 17%). We presumed that the reason for this is the more common dominancy of the left hemisphere than the right, with the ratio being about 20 : 1 or more [ 14]. Although there is a difference in the ratio, we thought that hemisphere dominancy and unilateral dominancy of the A1 artery may have some correlation. However, since nothing is known about it so far, future studies will need to investigate the relationship between A1 flow dominancy and hemisphere dominancy.
CONCLUSION
In this study, we found that A1 hypoplasia or aplasia itself did not affect the morphology or size of Acom aneurysms. Instead, when comparing the ruptured and unruptured groups, A1 hypoplasia or aplasia could contribute to the rupture of Acom aneurysms. We think that when the flow of one side of the A1 is restricted, this may affect the hemodynamics of the flow to the Acom aneurysm and increase the risk of aneurysm rupture.
Fig. 1.
Measurement methods for the morphology of aneurysm and H-complex variants in the study population. Symmetrical A1 is a case where both A1 arteries have no significant difference (A). In the case of hypoplastic A1, we defined it as A1 on one side with less than half of the diameter of the other side and less than 1 mm (B). Finally, aplastic A1 was defined as a case in which one side of the A1 was not visible on digital subtraction angiography (C). 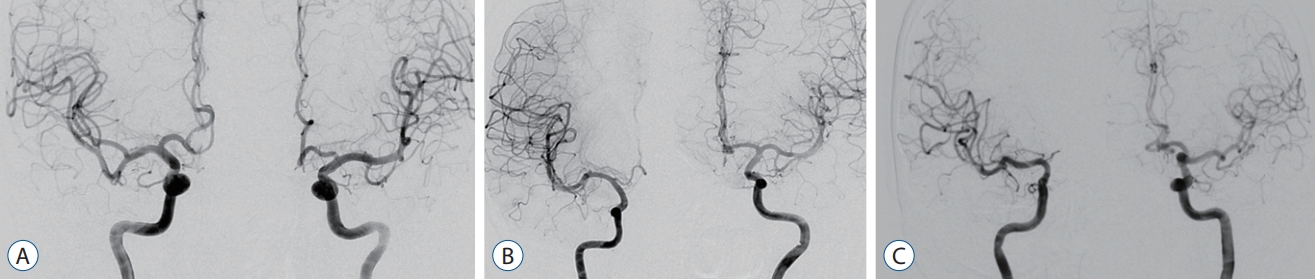
Fig. 2.
The method of measuring the size of aneurysms and each parameter used in this study. Neck width (n), dome width (d), height (h), the dome-to-neck ratio was calculated as d/n, the aspect ratio was calculated as h/n as described previously. Width of the A1 segment (w) was also measured. 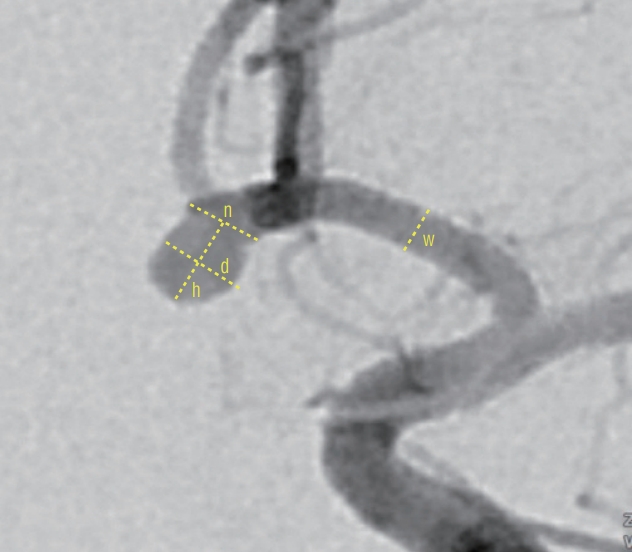
Table 1.
Baseline characteristics of the patients who underwent surgery for anterior communicating artery aneurysm
|
Total (n=209) |
Symmetric A1 (n=109) |
Hypoplastic or Aplastic A1 (n=100) |
p-value |
|
Age (years) |
59.80±11.15 |
60.77±11.15 |
58.75±11.11 |
0.191 |
|
Sex |
|
|
|
0.947 |
|
Male |
105 (50.2) |
55 (50.5) |
50 (50.0) |
|
|
Female |
104 (49.8) |
54 (49.5) |
50 (50.0) |
|
|
Smoking history |
|
|
|
0.274 |
|
No |
113 (54.1) |
55 (50.5) |
58 (58.0) |
|
|
Yes |
96 (45.9) |
54 (49.5) |
42 (42.0) |
|
|
Currently smoking |
|
|
|
0.661 |
|
No |
139 (66.5) |
71 (65.1) |
68 (68.0) |
|
|
Yes |
70 (33.5) |
38 (34.9) |
32 (32.0) |
|
|
Hypertension |
|
|
|
0.187 |
|
No |
105 (50.2) |
50 (45.9) |
55 (55.0) |
|
|
Yes |
104 (49.8) |
59 (54.1) |
45 (45.0) |
|
|
Hypertension medication (n=104) |
|
|
|
0.379 |
|
No |
17 (16.3) |
8 (13.6) |
9 (20.0) |
|
|
Yes |
87 (83.7) |
51 (86.4) |
36 (80.0) |
|
|
Diabetes |
|
|
|
0.784 |
|
No |
175 (83.7) |
92 (84.4) |
83 (83.0) |
|
|
Yes |
34 (16.3) |
17 (15.6) |
17 (17.0) |
|
|
Aneurysm size |
5.44±2.46 |
5.19±2.08 |
5.72±2.81 |
0.125 |
|
Daughter sac |
|
|
|
0.581 |
|
No |
92 (44.0) |
46 (42.2) |
46 (46.0) |
|
|
Yes |
117 (56.0) |
63 (57.8) |
54 (54.0) |
|
|
Aspect ratio |
1.51±0.76 |
1.43±0.53 |
1.59±0.94 |
0.125 |
|
Body mass index |
24.32±3.40 |
24.74±3.46 |
23.87±3.29 |
0.068 |
Table 2.
Demographic and radiologic difference of unruptured and ruptured groups
|
Total (n=209) |
Unruptured (n=107) |
Ruptured (n=102) |
p-value |
|
Age (years) |
59.80±11.15 |
62.84±9.42 |
56.62±11.95 |
<0.001 |
|
Sex |
|
|
|
0.946 |
|
Male |
105 (50.2) |
54 (50.5) |
51 (50.0) |
|
|
Female |
104 (49.8) |
53 (49.5) |
51 (50.0) |
|
|
Smoking history |
|
|
|
0.382 |
|
No |
113 (54.1) |
61 (57.0) |
52 (51.0) |
|
|
Yes |
96 (45.9) |
46 (43.0) |
50 (49.0) |
|
|
Currently smoking |
|
|
|
0.001 |
|
No |
139 (66.5) |
82 (76.6) |
57 (55.9) |
|
|
Yes |
70 (33.5) |
25 (23.4) |
45 (44.1) |
|
|
Hypertension |
|
|
|
0.111 |
|
No |
105 (50.2) |
48 (44.9) |
57 (55.9) |
|
|
Yes |
104 (49.8) |
59 (55.1) |
45 (44.1) |
|
|
Hypertension medication (n=104) |
|
|
|
<0.001 |
|
No |
17 (16.3) |
2 (3.4) |
15 (33.3) |
|
|
Yes |
87 (83.7) |
57 (96.6) |
30 (66.7) |
|
|
Diabetes |
|
|
|
0.331 |
|
No |
175 (83.7) |
87 (81.3) |
88 (86.3) |
|
|
Yes |
34 (16.3) |
20 (18.7) |
14 (13.7) |
|
|
Aneurysm size |
5.44±2.46 |
4.81±2.22 |
6.10±2.54 |
<0.001 |
|
Dome to neck ratio |
1.40±0.72 |
1.33±0.91 |
1.47±0.43 |
0.158 |
|
Aspect ratio |
1.51±0.76 |
1.40±0.85 |
1.62±0.63 |
0.041 |
|
Daughter sac |
|
|
|
<0.001 |
|
No |
92 (44.0) |
63 (58.9) |
29 (28.4) |
|
|
Yes |
117 (56.0) |
44 (41.1) |
73 (71.6) |
|
|
Morphology of A1 segment |
|
|
|
0.011 |
|
Symmetric A1 |
109 (52.2) |
65 (60.7) |
44 (43.1) |
|
|
Asymmetric A1 |
100 (47.8) |
42 (39.3) |
58 (56.9) |
|
|
Rt. sided hypoplasia |
83 (83.0) |
35 (42.1) |
48 (57.9) |
0.241 |
|
Lt. sided hypoplasia |
17 (17.0) |
7 (41.1) |
10 (58.9) |
0.198 |
|
Body mass index |
24.32±3.40 |
24.92±3.32 |
23.69±3.39 |
0.009 |
Table 3.
Multivariate analysis of risk factors related to subarachnoid hemorrhage
|
Variable |
OR (95% CI) |
p-value |
|
Age |
0.946 (0.914-0.979) |
0.001 |
|
Currently smoking |
|
|
|
No |
Reference |
|
|
Yes |
1.387 (0.667-2.882) |
0.381 |
|
Aneurysm size |
1.193 (1.023-1.390) |
0.024 |
|
Aspect ratio |
0.970 (0.626-1.501) |
0.890 |
|
Daughter sac |
|
|
|
No |
Reference |
|
|
Yes |
2.911 (1.503-5.638) |
0.002 |
|
Hypoplastic or aplastic A1 |
|
|
|
Symmetric A1 |
Reference |
|
|
Hypoplastic or aplastic A1 |
2.007 (1.055-3.820) |
0.034 |
|
Body mass index |
0.909 (0.827-0.999) |
0.047 |
References
1. Bonneville F, Sourour N, Biondi A : Intracranial aneurysms: an overview. Neuroimaging Clin N Am 16 : 371-382, 2006   2. Cebral JR, Castro MA, Burgess JE, Pergolizzi RS, Sheridan MJ, Putman CM : Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol 26 : 2550-2559, 2005   3. Chan V, Lindsay P, McQuiggan J, Zagorski B, Hill MD, O’Kelly C : Declining admission and mortality rates for subarachnoid hemorrhage in canada between 2004 and 2015. Stroke 50 : 181-184, 2019   4. Chen S, Mao J, Chen X, Li Z, Zhu Z, Li Y, et al : Association between body mass index and intracranial aneurysm rupture: a multicenter retrospective study. Front Aging Neurosci 13 : 716068, 2021    6. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al : Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 36 : 2773-2780, 2005   7. Grochowski C, Litak J, Kulesza B, Szmygin P, Ziemianek D, Kamieniak P, et al : Size and location correlations with higher rupture risk of intracranial aneurysms. J Clin Neurosci 48 : 181-184, 2018   8. Jeong YG, Jung YT, Kim MS, Eun CK, Jang SH : Size and location of ruptured intracranial aneurysms. J Korean Neurosurg Soc 45 : 11-15, 2009    9. Kang H, Ji W, Qian Z, Li Y, Jiang C, Wu Z, et al : Aneurysm characteristics associated with the rupture risk of intracranial aneurysms: a selfcontrolled study. PLoS One 10 : e0142330, 2015    10. Kayembe KN, Sasahara M, Hazama F : Cerebral aneurysms and variations in the circle of willis. Stroke 15 : 846-850, 1984   12. Krzyzewski RM, Tomaszewska IM, Lorenc N, Kochana M, Goncerz G, Klimek-Piotrowska W, et al : Variations of the anterior communicating artery complex and occurrence of anterior communicating artery aneurysm: A2 segment consideration. Folia Med Cracov 54 : 13-20, 2014  13. Marinković S, Kovacević M, Milisavljević M : Hypoplasia of the proximal segment of the anterior cerebral artery. Anat Anz 168 : 145-154, 1989  14. Medina LS, Bernal B, Ruiz J : Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: a bayesian analysis. Radiology 242 : 94-100, 2007   15. Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I, et al : Risk analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke 47 : 365-371, 2016   16. Perlmutter D, Rhoton AL Jr : Microsurgical anatomy of anterior cerebral anterior communicating recurrent artery complex. Surg Forum 27 : 464-465, 1976  18. Rinaldo L, McCutcheon BA, Murphy ME, Bydon M, Rabinstein AA, Lanzino G : Relationship of A1 segment hypoplasia to anterior communicating artery aneurysm morphology and risk factors for aneurysm formation. J Neurosurg 127 : 89-95, 2017   19. Rinaldo L, McCutcheon BA, Snyder KA, Porter AL, Bydon M, Lanzino G, et al : A1 segment hypoplasia associated with cerebral infarction after anterior communicating artery aneurysm rupture. J Neurosurg Sci 63 : 359-364, 2019   20. Rinkel GJ, Djibuti M, Algra A, van Gijn J : Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29 : 251-256, 1998   21. Suarez JI : Diagnosis and management of subarachnoid hemorrhage. Continuum (Minneap Minn) 21 : 1263-1287, 2015   22. Tarulli E, Fox AJ : Potent risk factor for aneurysm formation: termination aneurysms of the anterior communicating artery and detection of A1 vessel asymmetry by flow dilution. AJNR Am J Neuroradiol 31 : 1186-1191, 2010    23. Ujiie H, Liepsch DW, Goetz M, Yamaguchi R, Yonetani H, Takakura K : Hemodynamic study of the anterior communicating artery. Stroke 27 : 2086-2093, 1996   24. Ujiie H, Sato K, Onda H, Oikawa A, Kagawa M, Takakura K, et al : Clinical analysis of incidentally discovered unruptured aneurysms. Stroke 24 : 1850-1856, 1993  
|
|


















