Lee, Sohn, Chung, Park, Kim, and Lee: Clinical Analysis Comparing Efficacy between a Distal Filter Protection Device and Proximal Balloon Occlusion Device during Carotid Artery Stenting
Abstract
Objective
The main concern during transfemoral carotid artery stenting (CAS) is preventing cerebral embolus dislodgement. We compared clinical outcomes and intraprocedural embolization rates of CAS using a distal filter protection device or proximal balloon occlusion device.
Methods
From January 2011 to March 2015, a series of 58 patients with symptomatic or asymptomatic internal carotid artery stenosis вүҘ70% were treated with CAS with embolic protection device in single center. All patients underwent post-CAS diffusion-weighted magnetic resonance imaging (DW-MRI) to detect new ischemic lesions. We compared clinical outcomes and postprocedural embolization rates.
Results
CAS was performed in all 61 patients. Distal filter protection success rate was 96.6% (28/29), whose mean age was 70.9 years, and mean stenosis was 81%. Their preprocedural infarction rate was 39% (11/28). Subsequent DW-MRI revealed 96 new ischemic lesions in 71% (20/28) patients. In contrast, the proximal balloon occlusion device success rate was 93.8% (30/32), whose mean age was 68.8 years and mean stenosis was 86%. Preprocedure infarction rate was 47% (14/30). DW-MRI revealed 45 new ischemic lesions in 57% (17/30) patients. Compared with distal filter protection device, proximal balloon occlusion device resulted in fewer ischemic lesions per patient (p=0.028). In each group, type of stent during CAS had no significant effect on number of periprocedural embolisms. Only 2 neurologic events occurred in the successfully treated patients (one from each group).
Conclusion
Transfemoral CAS with proximal balloon occlusion device achieves good results. Compared with distal filter protection, proximal balloon occlusion might be more effective in reducing cerebral embolism during CAS.
Key Words: Carotid stenosis В· Diffusion magnetic resonance В· Embolic protection device В· Cerebral infarction.
INTRODUCTION
Carotid endarterectomy (CEA) is the gold standard for treating carotid stenosis. CEA has a low incidence of stroke and mortality but confers a high risk of morbidity, including myocardial infarction and cranial nerve injury. Carotid artery stenting (CAS) is rapidly becoming a popular alternative to CEA for treating carotid stenosis, and many reports suggest that it is more effective for treating high-risk CEA cases 6,16). However, the occurrence of intraprocedural embolization is a serious obstacle to more widespread CAS, which has a higher periprocedural stroke risk than CEA. Many studies have reported that embolic showers can occasionally cause permanent neurological deterioration 7,1215). Preventative embolic protection devices (EPDs) have significantly improved neurological prognosis 3,910,11). There are 2 general types of EPDs. Proximal balloon occlusion devices cause cessation of blood flow during the stenting procedure via simultaneous balloon occlusion of the external and common carotid artery 7,17). Distal filter protection devices capture debris with a filter that is placed in an artery distal to the lesion. However, no definite conclusion has been reached as to which device produces superior outcomes. In this study, we compared 2 different types of EPDs during CAS. We then, assessed the effectiveness of these devices using surrogate imaging endpoints obtained by diffusion-weighted magnetic resonance imaging (DW-MRI) performed on the first postprocedure day 5,1013,18).
MATERIALS AND METHODS
This retrospective, single-center study included patients with symptomatic and asymptomatic carotid stenosis who had been treated with CAS with either of 2 EPDs : a proximal balloon occlusion device or a distal filter protection device.
From January 2011 to March 2015, 61 patients with severe carotid lesions (symptomatic and asymptomatic) were selected for CAS with either Spider-FXв„ў (Covidien, Mansfield, MA, USA) or Mo.Ma Ultra (Medtronic, Santa Rosa, CA, USA) as the distal filter protection device or proximal balloon occlusion device, respectively. The inclusion and exclusion criteria and their adaptation for CAS were designed in accordance with the North American Symptomatic Carotid Endarterectomy Trial standards. Symptomatic carotid artery stenosis exceeded 50% and asymptomatic cases exceeded 70% of carotid stenosis rate 1). Passing the instrument through lesions was impossible in three patients, resulting in technical failure in, 1 patient in the distal filter protection device group and 2 patients in the proximal balloon occlusion device group. We used three different types of carotid artery stents : the ProtГ©gГ© EverFlexв„ў (ev3 Inc., Plymouth, MN, USA) as the open-cell type; Wallstent (Boston Scientific Corp., Natick, MA, USA) as the closed-cell type; and Cristallo Ideale (Invatec, Roncadelle, Italy) as the mixed-cell type.
All patients were premedicated with clopidogrel 75 mg/day and aspirin 100 mg/day for at least 3 days before the intervention. Clopidogrel was continued for at least 1 month after CAS, and aspirin was continued indefinitely. Beta blockers were discontinued at least 24 hours before the procedure, and other medications were continued at the discretion of the referring physician.
Postprocedure MRI was performed in all patients. When neurological symptoms occurred after a procedure, MRI was performed immediately. Otherwise, MRI was performed on the first postprocedure day to confirm any new cerebral ischemic lesions, even if patients were asymptomatic.
Fluid-attenuated inversion recovery (FLAIR) and DW-MRI images were captured using a 3.0T machine (Magnetom Skyra, Siemens, Germany). DW-MRI images were obtained with the following parameters : b values of 1000 s/mm2; repetition time (TR)/echo time (TE)/excitation, 5150 ms/64 ms/1; matrix 150Г—150; field of view (FOV) 220Г—220 mm; section thickness 5 mm; interslice gap 1.0 mm; and total acquisition time, 2 minutes 19 seconds. The FLAIR images were obtained as follows : TR/TE/excitation, 6510 ms/190 ms/1; inversion time 2127 ms; matrix 512Г—251; FOV 220Г—220 mm; section thickness 5 mm; intersection gap 1.0 mm; and total acquisition time 2 minutes 49 seconds. FLAIR images, DW-MRI, and apparent diffusion coefficient maps were used to identify new cerebral ischemic lesions. The number of isolated lesions was counted. All imaging was performed at the our hospital and analyzed by the neurosurgeon who performed the CAS procedure.
Neurologic events were divided into the following categories : transient ischemic attack (TIA) (symptoms of neurologic deficit improving within 24 hours); minor stroke [symptoms of neurological deficit continuing after 24 h, National Institute of Health Stroke Scale (NIHSS) score вүӨ4]; and major stroke (symptoms of neurological deficit continuing after 24 hours, NIHSS score вүҘ5) 8). Student's t-test, Pearson's chi-squared test, and Fisher's exact test were used to analyze and compare the clinical characteristics of the 2 groups. Fisher's exact test was used to examine embolization-related complications, and Pearson's chi-squared test and the Mann-Whitney U test were used to examine the rate and amount of positive results as seen on postprocedure DW-MRI. Two-way analysis of variance with the post hoc Tukey method was used for statistical analysis of stent influence. A p value<0.05 was considered statistically significant.
RESULTS
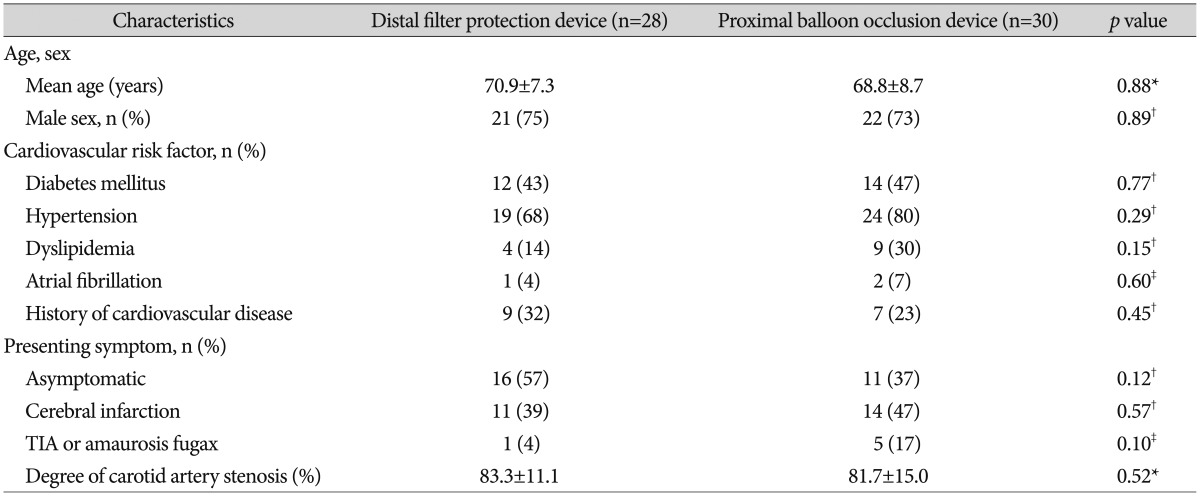
The clinical characteristics of patients in both groups are summarized in Table 1. No significant differences were found between the groups with regard to patient demographics (age, sex); cardiovascular risk factors (diabetes mellitus, hypertension, dyslipidemia, atrial fibrillation, and history of cardiovascular disease); presenting symptoms (including cerebral infarction, TIA or amaurosis fugax, or asymptomatic); or degree of carotids artery stenosis (83Вұ11% vs. 81Вұ15%, p=0.52). In the distal filter protection device and proximal balloon occlusion device groups, clinical and procedural success was achieved in 96.6% and 93.8% of patients, respectively, and instruments failed to pass through lesions in 3 patients (1 in the distal filter protection device group and 2 in the proximal balloon occlusion device group). Postprocedure lesions were observed on DW-MRI in 20 patients (71%) in the distal filter protection device group and 17 (57%) in the proximal balloon occlusion device group, with no significant difference between groups ( p=0.242). The number of patients who had 1, 2, and вүҘ3 DWI-positive lesions was 3 (10.1%), 2 (7.2%), and 15 (53.6%) in the distal filter protection device group and 4 (13.3%), 6 (20.0%), and 7 (23.3%) in the proximal balloon occlusion device group, respectively, with no significant difference between the 1 or 2 lesion group ( p=0.760 and p=0.141, respectively). However, in the вүҘ3-lesion group, the number of patients was significantly lower in the proximal balloon occlusion device group ( p=0.018). The number of ischemic lesions per CAS patient was significantly lower in the proximal balloon occlusion device group than in the distal filter protection device group (4.8 vs. 2.7, respectively, p=0.028). In the ipsilateral subgroup, the number of lesions was lower in the proximal balloon occlusion device group, but not significantly ( p=0.096). In the contralateral and posterior fossa subgroups, the number of lesions was lower in the proximal balloon occlusion device group, but not significantly ( p=0.063) ( Table 2). Ischemic neurologic events were observed in 2 patients (3.6%) in the entire study population. One patient in the distal filter protection device group experienced a major middle cerebral artery infarction with aphasia and hemiparesis the day after the procedure. One patient in the proximal balloon occlusion device group had a TIA. No other significant intergroup differences in the incidence of ischemic neurologic events were observed ( p=1.00) ( Table 3).
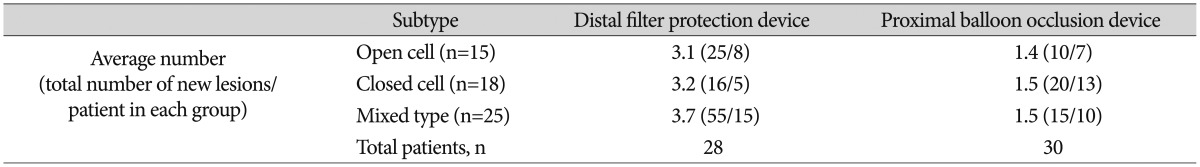
Table 4 shows the number of new postprocedural lesions per patient, as seen on DW-MRI, using 3 types of stents : open cell, closed cell, and mixed type. No interaction between CAS and EPD was found ( p=0.206), and postprocedural number of new lesions had no statistically significant relation to type of carotid artery stent used ( p=0.588). However, EPD type significantly affected the number of postprocedural lesions observed ( p=0.030, Tukey method).
DISCUSSION
In a study by Kastrup et al. 10) comparing the outcomes of CAS with and without an EPD, the incidence of both minor and major stroke was significantly lower when an EPD was used. The authors concluded that embolization-related complications rates were reduced (to 5%) by using an EPD during CAS after a prospective protocol. Our study found a lower stroke rate using both a distal filter protection device and a proximal balloon occlusion device [3.5% (1/28 patients) vs. 3.3% (1/30 patients), respectively]. The recent Prevention of Cerebral Embolization by Proximal Balloon Occlusion Compared to Filter Protection During Carotid Artery Stenting (PROFI) study reported by Bijuklic et al. 3) randomly compared distal filter protection device with proximal balloon occlusion devices and found that incidence of new cerebral ischemic lesions was significantly different between devices (87.1% vs. 45.2%, respectively) 4). Our study showed a higher incidence of new cerebral ischemic lesions in the distal filter protection device group than in the proximal balloon occlusion device group (71% vs. 57%, respectively), but this difference was not statistically significant. However, the number of lesions per patient was significantly higher in the distal filter protection device group than in the proximal balloon occlusion device group ( p=0.028). In addition, in the distal filter protection device group, the proportion of patients who developed вүҘ3 lesions as seen on DW-MRI reached 54% (15/28 patients), significantly greater than that in the proximal balloon occlusion device group (23%, 7/30 patients) ( p=0.018). Stabile et al. 17) have reported the superior efficacy of the proximal balloon occlusion device for embolic protection. The proximal balloon occlusion device provides neuroprotection through all phases of the CAS procedure, including initial lesion crossing, whereas distal filter protection devices provide neuroprotection after crossing the lesion or allow particles smaller than their pore size to pass through. In addition, a proximal balloon occlusion device can capture particulate debris with higher efficiency. Furthermore, Bijuklic et al. 3) reported that distal filter protection device can become overloaded with debris, thus posing the risk of spilling the contents of the filter during retrieval, which can occasionally be difficult. In an in vitro study, MГјller-HГјlsbeck et al. 14) reported that the closed-cell stent design resists particle penetration not only because of its smaller cell size, which does not allow penetration of embolic particles, but also because it has a highly conformable supporting structure. However, no studies have documented an association between stent type and the number of periprocedural embolic events in vivo. Our study clinically showed that the type of stent used does not significantly affect the average number of postprocedural lesions observed per patient. In 2011, Voeks et al. 20) reported that especially age and sex are important risk factors for postprocedural stroke after CAS. In our study, there was no significant difference in age ( p=0.88) or sex ( p=0.89) as risk factors; in addition, similar demographic data, preprocedure symptomatology, and degree of stenosis were observed, without statistical difference ( Table 1). Thus, the frequency of periprocedural adverse events was compared between the 2 embolic protection groups under similar conditions. Study limitations include the fact that this was not a randomized study. The initial series of patients was treated with the distal filter protection device from January 2011 to July 2013, and the subsequent series of patients was treated with the proximal balloon occlusion device from August 2013 to March 2015, following favorable initial outcomes with the latter device. Therefore, statistical error including selection bias and generalizability may be expected.
Another limitation of this study was that plaque characteristics were not investigated or reported because carotid ultrasonography and/or cervical MRI were not performed. The high-risk group such as patients with unstable, ulcerative, or heterogeneous plaque or those with intraplaque hemorrhage or intraluminal thrombus have a high prevalence of stroke after CAS 2,19). Therefore, plaque characteristics should be considered in both groups. These limitations need to be addressed in future investigations. Major advances in the field of CAS include the introduction of appropriate dedicated stents, better patient selection, improvement in physician expertise, and new cerebral protection devices. Although EPDs have significantly contributed to progress in carotid artery interventions, CAS still carries the inherent risk of stroke. Further studies on EPDs are required.
CONCLUSION
The number of postprocedure ischemic lesions per patient and the incidence of ischemic lesions as seen on DW-MRI were lower in a patients treated with CAS using proximal balloon occlusion device. Compared with distal filter protection device, proximal balloon occlusion device might be more effective in reducing cerebral embolism during CAS.
References
1. Barnett HJM : NASCET CollaboratorsFinal results of the North American Symptomatic Carotid Endarterectomy Trial (nascet). Stroke 1998, 29 : 286,
2. Biasi GM, Froio A, Diethrich EB, Deleo G, Galimberti S, Mingazzini P, et al : Carotid plaque echolucency increases the risk of stroke in carotid stenting : the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004, 110 : 756-762,   3. Bijuklic K, Wandler A, Hazizi F, Schofer J : The PROFI study (Prevention of Cerebral Embolization by Proximal Balloon Occlusion Compared to Filter Protection During Carotid Artery Stenting) : a prospective randomized trial. J Am Coll Cardiol 2012, 59 : 1383-1389,   4. Cohen SA, Snead D, Ouriel K, Fayad P, Katzen B, Wholey M, et al : Effectiveness of embolic protection in capturing debris and reducing 30-day stroke rates during carotid stenting. Circulation 2005, 112 : U476,
5. El-Koussy M, Schroth G, Do DD, Gralla J, Nedeltchev K, von Bredow F, et al : Periprocedural embolic events related to carotid artery stenting detected by diffusion-weighted MRI : comparison between proximal and distal embolus protection devices. J Endovasc Ther 2007, 14 : 293-303,   6. European Stroke OrganisationTendera M, Aboyans V, Bartelink ML, Baumgartner I, ClГ©ment D, et al : ESC Guidelines on the diagnosis and treatment of peripheral artery diseases : Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries : the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011, 32 : 2851-2906,   7. Hauth EA, Jansen C, Drescher R, Schwartz M, Forsting M, Jaeger HJ, et al : MR and clinical follow-up of diffusion-weighted cerebral lesions after carotid artery stenting. AJNR Am J Neuroradiol 2005, 26 : 2336-2341,   8. Iko M, Tsutsumi M, Aikawa H, Matsumoto Y, Go Y, Nii K, et al : Distal protection filter device efficacy with carotid artery stenting : comparison between a distal protection filter and a distal protection balloon. Jpn J Radiol 2013, 31 : 45-49,   9. Kastrup A, NГӨgele T, GrГ¶schel K, Schmidt F, Vogler E, Schulz J, et al : Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke 2006, 37 : 2312-2316,   10. Kastrup A, Skalej M, Krapf H, NГӨgele T, Dichgans J, Schulz JB : Early outcome of carotid angioplasty and stenting versus carotid endarterectomy in a single academic center. Cerebrovasc Dis 2003, 15 : 84-89,   11. Macdonald S, Evans DH, Griffiths PD, McKevitt FM, Venables GS, Cleveland TJ, et al : Filter-protected versus unprotected carotid artery stenting : a randomised trial. Cerebrovasc Dis 2010, 29 : 282-289,   12. Maggio P, Altamura C, Landi D, Migliore S, Lupoi D, Moffa F, et al : Diffusion-weighted lesions after carotid artery stenting are associated with cognitive impairment. J Neurol Sci 2013, 328 : 58-63,   13. Montorsi P, Caputi L, Galli S, Ciceri E, Ballerini G, Agrifoglio M, et al : Microembolization during carotid artery stenting in patients with high-risk, lipid-rich plaque. A randomized trial of proximal versus distal cerebral protection. J Am Coll Cardiol 2011, 58 : 1656-1663,   14. MГјller-HГјlsbeck S, SchГӨfer PJ, Charalambous N, Schaffner SR, Heller M, Jahnke T : Comparison of carotid stents : an in-vitro experiment focusing on stent design. J Endovasc Ther 2009, 16 : 168-177,   15. Rapp JH, Wakil L, Sawhney R, Pan XM, Yenari MA, Glastonbury C, et al : Subclinical embolization after carotid artery stenting : new lesions on diffusion-weighted magnetic resonance imaging occur postprocedure. J Vasc Surg 2007, 45 : 867-872, discussion 872-874   16. SPACE Collaborative GroupRingleb PA, Allenberg J, BrГјckmann H, Eckstein HH, Fraedrich G, et al : 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients : a randomised non-inferiority trial. Lancet 2006, 368 : 1239-1247,   17. Stabile E, Salemme L, Sorropago G, Tesorio T, Nammas W, Miranda M, et al : Proximal endovascular occlusion for carotid artery stenting : results from a prospective registry of 1,300 patients. J Am Coll Cardiol 2010, 55 : 1661-1667,   18. Taha MM, Maeda M, Sakaida H, Kawaguchi K, Toma N, Yamamoto A, et al : Cerebral ischemic lesions detected with diffusion-weighted magnetic resonance imaging after carotid artery stenting : comparison of several anti-embolic protection devices. Neurol Med Chir (Tokyo) 2009, 49 : 386-393,   19. Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, et al : Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques : a high-resolution magnetic resonance imaging study. Circulation 2005, 111 : 2768-2775,   20. Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Sternbergh WC 3rd, et al : Age and outcomes after carotid stenting and endarterectomy : the carotid revascularization endarterectomy versus stenting trial. Stroke 2011, 42 : 3484-3490,   
TableВ 1
Baseline characteristics of patients who underwent CAS using an embolic protection device with an distal filter protection device or proximal balloon occlusion device
TableВ 2
MRI findings after CAS with distal filter protection devices or proximal balloon occlusion devices
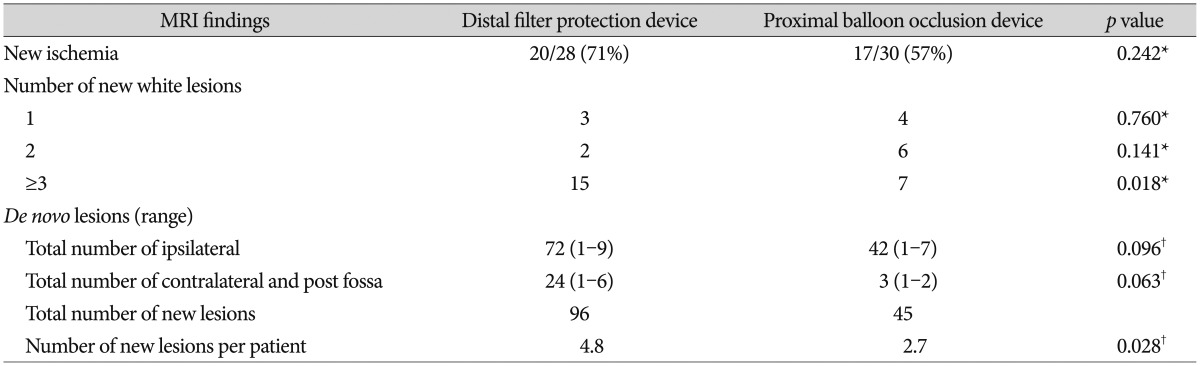
TableВ 3
Technical success rate and neurologic events following CAS with distal filter protection devices or proximal balloon occlusion devices

TableВ 4
Average number of and proportion of patients with new lesions following CAS with distal filter protection devices or proximal balloon occlusion devices
|
|



















