Kim, Kim, and Kong: Intra-arterial and Intravenous Tirofiban Infusion for Thromboembolism during Endovascular Coil Embolization of Cerebral Aneurysm
Abstract
Objective
Thromboembolism is the one of the most serious complications that can occur during endovascular coil embolization of cerebral aneurysm. We report on the effectiveness and safety of intra-arterial/intravenous (IA/IV) glycoprotein IIb/IIIa inhibitor (tirofiban) infusion for treating thromboembolism during endovascular coil embolization of cerebral aneurysm.
Methods
We performed a retrospective analysis of 242 patients with ruptured or unruptured cerebral aneurysms (n=264) who underwent endovascular coil embolization from January 2011 to June 2014. Thromboembolism occurred in 20 patients (7.4%), including 14 cases of ruptured aneurysms and 6 cases of unruptured aneurysms. The most common site of aneurysms was the anterior communicating artery (n=8), followed by middle cerebral artery (n=6). When we found an enlarged thromboembolism during coil embolization, we tried to dissolve it using tirofiban administered via IA and IV loading (5 μg/kg, respectively) for 3-5 minutes followed by IV maintenance (0.08 μg/kg/min) for approximately 4-24 hours.
Results
In 4 of 5 patients with total vessel occlusion, the vessel was recanalized to Thrombolysis in Cerebral Infarction Perfusion Scale (TICI) grade 3, and in 1 patient to TICI grade 2a. In 2 patients with partial vessel occlusion and 13 patients with minimal occlusion, the vessel recanalized to TICI grade 3. Irrelevant intracerebral hemorrhage was noted in 1 patient (5%), and thromboemboli-related cerebral infarction developed in 5 patients (25%), of which only 1 (5%) was symptomatic.
Conclusion
IA/IV infusion and IV maintenance with tirofiban appear to be an effective rescue treatment for thromboembolism during endovascular coil embolization in patients with ruptured or unruptured cerebral aneurysms.
Key Words: Endovascular coil embolization · Thromboembolism · Tirofiban · Intra-arterial/intravenous infusion.
INTRODUCTION
Thromboembolic complications that develop during endovascular coil embolization to treat cerebral aneurysms are the most serious and dangerous ones because they can result in serious neurological sequelae 6,8,9,24). Their incidence has been reported as approximately 6-7% 3,25). There are several methods for dissolving thromboembolism, including intravenous (IV) heparin, IV aspirin, thrombolysis using urokinase or tissue plasminogen activator, and mechanical thrombectomy. These methods all have attendant risks, including accompanying hemorrhage, so the medications used should have easy applications, have a short half-life, confer low bleeding tendency, and so forth. Recently, glycoprotein (GP) IIb/IIIa inhibitors have been used to treat thromboembolism that develops during endovascular coil embolization for cerebral aneurysm because they may fulfill these criteria 1). There are 2 types of GP IIb/IIIa inhibitors: reversible ones (tirofiban and eptifibatide) and an irreversible one (abciximab). In treating thromboembolism, 2 infusion routes are available for GP IIb/IIIa inhibitors: intra-arterial (IA) and IV. Systemic IV administration of tirofiban was the first method introduced, and its efficacy has been proven in patients with myocardial infarction. The IA administration of tirofiban was introduced later, its advantages being the focal elevation of tirofiban concentration at the lesion, decreased total dose of tirofiban and low incidence of hemorrhage. However, Brinjikji et al. 3) reported no differences in terms of clinical outcomes and complication rates between the 2 administration routes. We combined the 2 routes of administration, IA and IV infusion, of the reversible GP IIb/IIIa inhibitor, tirofiban to study their advantages and disadvantages for elevating focal concentration and systemic concentration of the drug at the same time. Here, we present the effectiveness and safety of combining infusion routes of tirofiban for treating thromboembolism during endovascular coil embolization.
MATERIALS AND METHODS
Patient characteristics
This retrospective analysis was performed in 242 patients with ruptured or unruptured cerebral aneurysms (n=264) who underwent endovascular coil embolization from January 2011 to June 2014 at CHA Bundang Medical Center. This study was approved by the Institutional Review Board (IRB) of CHA Bundang Medical Center, CHA Univeristy School of Medicine on Aug 18th, 2016 with a waiver of informed consent (IRB No. BD2015-135). The subarachnoid hemorrhage of the ruptured aneurysm was noted on a computed tomography (CT) scan of the brain, or rarely, on a brain magnetic resonance imaging (MRI). In most cases, the unruptured aneurysm was noted on brain magnetic resonance angiography or CT angiography. The aneurysms (ruptured and unruptured) were confirmed with conventional cerebral angiography. Thromboembolism developed during the endovascular coil embolization procedure in 20 patients (7.57%): 14 cases of ruptured aneurysms and 6 cases of unruptured aneurysms. Patient age ranged from 33 to 76 years (mean±standard deviation [SD]=55.75±12.12 years), and there were 12 men and 8 women. The maximum diameter of the aneurysms ranged from 2.4 mm to 12.5 mm (mean±SD=6.19±2.68 mm), and 16 aneurysms (80%) were wide-necked, in which the dome-to-neck ratio was less than 2.0. The most common site for the aneurysms was the anterior communicating artery (n=8), followed by the middle cerebral artery (n=6). Each patient’s clinical status was evaluated before and after the procedure using the modified Rankin Scale (mRS).
Coil embolization and tirofiban infusion
Patients with unruptured aneurysms were given an oral regimen of dual antiplatelets (aspirin and clopidogrel) for at least 5 days; alternatively, oral antiplatelets (aspirin 300 mg and clopidogrel 300 mg) were given as a loading dose the day before the procedure. Endovascular coil embolization was performed under general endotracheal anesthesia. We performed endovascular coil embolization under high-resolution digital roadmapping in a biplane angiographic unit. Systemic heparinization was done during the procedure; initial heparinization (loading dose of 1500-2000 IU and maintenance dose of 700-800 IU/hour) was started when the guiding catheter was inserted in the unruptured aneurysms, and when some filling of the aneurysmal sac occurred in the ruptured aneurysms. In this series, stent-assisted coil embolizations were performed in 9 patients using either Solitaire stents (Covidien, Plymouth, MN, USA) or Enterprise stents (Cordis Neurovascular, Miami Lakes, FL, USA). Of these 9 patients, in 6 patients, a single Solitaire stent was used; in 2 patients (who had a blood blister-like aneurysm), double stents (solitaire and enterprise) were used; and in 1 patient, the Solitaire stent had to be retrieved owing to thrombus formation. Balloon-assisted coil embolization was performed in 1 patient, and the double-catheter technique was used in another patient.
When we found an enlarged thromboembolism during coil embolization, we tried to dissolve it using tirofiban and continued the coil embolization. However, in most cases, the thromboembolism occurred at the end of the coil embolization procedure, so we were able to finish the procedure by dissolving the thromboemboli. To accomplish this, we diluted tirofiban to a concentration of 25 μg/mL and gave an IA (via the guiding catheter or microcatheter)/IV loading dose at the same time as a 5 μg/kg dose, respectively (~80% of the usual recommended). IV tirofiban maintenance therapy was given at a dose of 0.08 μg/kg/min (~80% of the usual recommended dose) for 4-24 hours. In patients who underwent stent-assisted coil embolization of ruptured aneurysms, after full filling of the aneurysmal sac, oral antiplatelets (aspirin 300 mg and clopidogrel 300 mg) were given in a loading dose, and IV maintenance therapy continued for 4 hours, after which the dose was gradually discontinued for several hours (for example, reduction by 8-10% per hour and discontinuance at the dosage of about 50%). In the other aneurysms, IV maintenance therapy continued for 24 hours and the dose was gradually discontinued. Dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) was maintained for at least 3 months in patients who had undergone stent-assisted coil embolization and was then switched to antiplatelet monotherapy (aspirin 100 mg). In 4 patients, mechanical thrombectomy with a microcatheter and microwire was performed together with the IA/IV tirofiban infusion and IV maintenance. In most patients, systemic heparinization was not maintained after the procedure, except in cases of distal embolism.
Effectiveness of the IA/IV tirofiban infusion and IV maintenance therapy
When discussing thromboembolic complications, we defined the term “proximal thrombus” as a thrombus located proximally to the aneurysmal neck, “neck thrombus” as located in the area of the neck, “distal thrombus” as located distally to the aneurysmal neck and “embolus” as located within different vascular territory from the aneurysm. Of the 20 patients in whom thromboembolism developed during the endovascular coil embolization procedure, proximal thrombus occurred in 4 (20%), neck thrombus occurred in 14 (70%), distal thrombus occurred in 1 (5%) and embolus and neck thrombus simultaneously occurred in 1 (5%). The origin of the thrombi related to coil protrusion in 11 cases (55%), to in-stent thrombus in 4 cases (20%), to both coil protrusion and in-stent thrombus in 3 cases (15%), to catheter displacement in 1 case (5%) and to simultaneous emboli from the carotid bulb and the coil protrusion in 1 case (5%).
Blood flow was evaluated using angiography and measured using Thrombolysis in Cerebral Infarction perfusion scale (TICI) ( Table 1) 12). A postprocedural brain CT was obtained within 3 hours of the procedure to evaluate cerebral hemorrhage or infarction; an additional brain CT or MRI was obtained from patients who had neurological deterioration. Hemorrhage or infarction was evaluated for its relationship to thromboembolism by examining vascular territory, timing, vasospasm and other factors.
Data analysis
Imaging studies, including brain CT, MRI, and cerebral angiography, were evaluated by 2 neurointerventionists (1 neuroradiologist and 1 neurosurgeon). These experts graded blood flow by TICI scale and checked cerebral hemorrhage or infarction for any association with thromboembolism. Other neurosurgeons reviewed the written patient charts.
RESULTS
Clinical and radiographical characteristics are described in Table 2. The postprocedural mRS score on day 1 showed no change from preprocedural mRS scores, except for 1 case (in which score changed from 2 to 4). In this patient, stent-assisted endovascular coil embolization of a right anterior cerebral artery, A2-3 aneurysm was performed, but severe vasospasm and thrombus occurred in right A2. Therefore, it was not possible to avoid the patient’s cerebral infarction and neurological deterioration. The most common origin of thrombus formation was coil protrusions, seen in 15 cases (75%). These cases comprised 12 cases (60%) of simple coil protrusion and 3 cases (15%) of stent-assisted coil protrusion. The stent was the cause in 4 cases (20%), 2 of which had double stents (2 cases), 1 of which had a small parent vessel, and 1 of which had severe vasospasm. Microcatheter displacement caused thrombus formation in 1 patient (5%). Embolism from carotid plaque was the cause of thrombus formation in 1 patient, occurring in the presence of simultaneous coil protrusion.
Thromboembolism developed during endovascular coil embolization in 20 out of 264 cases of aneurysm (7.57%). Of these 20 cases, 5 were TICI grade 0, 2 were TICI grade 1, 7 were TICI grade 2a, and 6 were TICI grade 2b. In the 5 cases that were TICI grade 0, 1 achieved TICI grade 2a after recanalization, and 4 cases improved to TICI grade 3 after recanalization ( Fig. 1, Table 3). Two cases that were TICI grade 1 achieved TICI grade 3 after recanalization ( Fig. 2), and 13 cases that were TICI grade 2a, and 2b achieved TICI grade 3 after recanalization. The patient with a simultaneous embolus from a carotid bulb plaque improved from TICI grade 1 to grade 2 after recanalization ( Table 3). Delayed irrelevant intracerebral hemorrhage was noted in 1 patient (5%) two weeks after the procedure, and thromboemboli-related (or procedure-related) cerebral infarction developed in 5 patients (25%), of which only 1 patient was symptomatic.
DISCUSSION
Thromboembolic events are the most common complications during endovascular coil embolization of cerebral aneurysm and have proven challenging to neurointerventionists. Possible causes of thrombus formation are flow disturbances and the effects of the procedure itself. Flow disturbances can occur at the coil surface or the interface between coil and vessel wall; they can be caused by an irregular coil surface or coil protrusions into the vessel lumen, resulting in vessel narrowing or vasospasm. Procedure-related causes include intimal injury from microcatheter displacement or stent deployment, in-stent thrombus or migration of a preexisting intra-aneurysmal thrombus 4,5). In our patients, the most common cause of thrombus formation was coil protrusions (simple coil protrusion: 60%, stent-assisted coil protrusion: 15%), findings that are consistent with previous reports 4,5). Thromboembolic events also have been associated with wide-necked aneurysms 20,24). In our study, the mean dome-to-neck ratio of the aneurysms was 1.56±0.89 (0.73-4.71), and 16 patients had a wide-necked aneurysm (<2.0 dome-to-neck ratio), among which thromboembolic events occurred in four patients. Two classes of medications are used for treating thromboembolism during endovascular coil embolization: thrombolytic agents (a tissue plasminogen activator or urokinase) and GP IIb/IIIa inhibitors. Cronqvist et al. 7) reported on the outcomes of thrombolysis using urokinase in 19 patients and combining thrombolysis with mechanical thrombus fragmentation in 9 patients. In their report, results as seen on angiography were superb: complete recanalization was achieved in 10 patients, and partial recanalization was achieved in 9 patients. Clinical outcomes were also positive: a good clinical outcome was obtained in 14 patients, of whom 9 showed complete recanalization and 5 showed partial recanalization. However, significant hemorrhagic complications occurred in 3 patients. Koebbe et al. 16) previously reported on the outcome of thrombolysis using urokinase or tissue plasminogen activator in 5 patients, 2 of whom experienced significant subarachnoid hemorrhage. In these studies, although thrombolysis using urokinase or tissue plasminogen activators effectively led to vessel recanalization, the rates of significant hemorrhage were high. Hyperacute thrombi that develop during endovascular coil embolization consist of platelet aggregates that are not yet stabilized by fibrin cross-linking. Theoretically, GP IIb/IIIa inhibitors reversibly antagonize fibrinogen binding to the GP IIb/IIIa receptors of platelets and inhibit platelet aggregation in order to prevent thrombi from forming and propagating. Therefore, many clinicians try to dissolve thrombi during endovascular coil embolization using GP IIb/IIIa inhibitors. Fiorella et al. 10) reported on 13 patients with thromboembolism during endovascular embolization who were treated with a GP IIb/IIIa inhibitor (abciximab). Although tissue plasminogen activator was simultaneously administered to 5 of these patients, 7 patients achieved complete recanalization, 6 patients achieved partial recanalization, and no new hemorrhagic complications developed. In another report, 52 of 54 patients showed complete or partial dissolution of their thrombi, and no new hemorrhagic complications developed 11). In a recent meta-analysis by Brinjikji et al. 2,3), the GP IIb/IIIa inhibitors abciximab, tirofiban, and eptifibatide were shown to be superior to thrombolytics in terms of recanalization rate (72% vs. 50%), perioperative morbidity (11% vs. 29%) and long-term morbidity (16% vs. 35%). Abciximab is a monoclonal antibody with a large molecular weight. It binds irreversibly to the GP IIb/IIIa receptor at the β-chain of the integrin. Tirofiban is a nonpeptide tyrosine derivative with a small molecular weight based on a disintegrin polypeptide. Tirofiban reversibly and competitively antagonizes fibrinogen and von Willebrand factor binding to the GP IIb/IIIa receptors of platelets, and thereby inhibits platelet aggregation. Correspondingly, tirofiban’s potency for dissolving thromboemboli is inferior to abciximab’s, but its half-life is significantly shorter (1.5-2.0 hours for tirofiban vs. ≤48 hours for abciximab) 11,25). In addition, with tirofiban, bleeding time normalizes within 4 hours after discontinuation compared with 12 hours for abciximab 11). Many authors have reported that abciximab is safe to use as a rescue treatment for intraprocedural/periprocedural thrombi that develop during endovascular embolization 10,18,19,21). However, in theory, the affinity of abciximab to GP IIb/IIIa receptors is higher than tirofiban’s, and the half-life of tirofiban is usually much shorter than that of abciximab. Thus, the incidence of perioperative or postoperative hemorrhage is likely to be higher in patients treated with abciximab. Some authors have expressed concerns that abciximab could result in significant hemorrhagic complications 22,23,25); other authors preferred tirofiban for treating patients who needed decompressive surgery and extraventricular drainage after undergoing endovascular coil embolization of cerebral aneurysm 4). In contrast, Jeong and Jin 14) reported that both abciximab and tirofiban exhibited similar safety and vessel recanalization rates. Recently, the meta-analysis by Brinjikji et al. 2,3) compared patients receiving abciximab with those receiving tirofiban (and eptifibatide). In their report, patients receiving tirofiban (and eptifibatide) had significantly higher recanalization rates (83% for tirofiban vs. 66% for abciximab; p=0.05), but there was no difference in clinical outcomes between the 2 groups (postoperative hemorrhage rate; 14% for tirofiban vs. 7% in abciximab; p=0.12) 3). Tirofiban has been used in patients with acute myocardial infarction, usually with 1 of 2 regimens. The first regimen is an IV loading dose of 0.4 μg/min/kg for 30 minutes followed by an IV maintenance dose of 0.1 μg/min/kg for over 48 hours. The second regimen is an IV loading dose of 25 μg/kg for 3-5 minutes followed by an IV maintenance dose of 0.15 μg/min/kg for more than 48 hours 17). When tirofiban began to be used for treating thromboemboli during endovascular coiling, the first regimen was used: an IV loading dose of 0.4 μg/min/kg for 30 minutes followed by an IV maintenance dose of 0.1 μg/min/kg for more than 48 hours. This regimen proved effective for dissolving thromboemboli during endovascular coil embolization 4). Recently, another regimen was introduced for treating thromboemboli during endovascular coiling. Tirofiban was repeatedly given in a loading dose (~4-8 μg/kg or 300 μg) via the IA route of administration for several minutes until the thromboemboli were dissolved, as confirmed on repeated cerebral angiography. IV maintenance therapy was then applied in a few cases. It is reported that this regimen was possibly able to elevate the focal concentration of tirofiban near the thromboemboli and thereby reduce the total dosage of tirofiban 5,13,15). Cho et al. 5) reported 87.2% of recanalization rates and no postoperative hemorrhages with IA tirofiban doses of 0.25 to 1.25 mg and Jeon et al. 13) reported 80% of recanalization rates and no postoperative hemorrhages with IA tirofiban doses of 0.3 to 1.2 mg. Many authors believe that both IA and IV administration of GP IIb/IIIa inhibitors is effective for dissolving thrombi during endovascular coil embolization. However, some authors believe that IA administration is better than the IV route for 2 reasons. First, the IA route can elevate the focal concentration of the drug with its rapid-dissolving action. Second, this dissolving action can be achieved with a relatively low dose of the drug and leads to a low rate of hemorrhagic complications 11). Fiorella reported that the time it took for GP IIb/IIIa inhibitors to dissolve thrombi was less than 5 minutes via the IA route, but more than 10 minutes for the IV route 10). However, the meta-analysis by Brinjikji et al. 3) compared patients receiving GP IIb/IIIa inhibitors by IA route with those by IV route. In their report, there was no difference in recanalizaton rates (77% for IA route vs. 70% for IV route; p=0.36) and postoperative hemorrhage rates (5% for IA route vs. 7% for IV route; p=0.66) between the 2 groups. Although the elevation of the focal concentration of tirofiban may be help for dissolving thrombus, we believed that tirofiban will operate above some systemic concentration like other drugs and we doubted that IA administration of the drug could focally elevate the drug’s concentration, resulting in more effective thrombus dissolution. If this were true, we reasoned, then such a focal elevation of the drug’s concentration could be dangerous and cause hemorrhagic lesions, such as a ruptured aneurysm. In addition, the repeated IA loading methods depends somewhat on the clinician’s experience, specially in point of drug dosage because the drug dosage is not specific. We would like to choose more standardized methods specially in terms of dosage and administration routes.
Because of this reason, we combined the 2 existing administration routes. We performed simultaneous IA and IV loading with approximately 80% (5 μg/kg, respectively) of the standard recommended dosage (i.e., 12 μg/kg) of tirofiban used in myocardial infarction patients for 3-5 minutes. Subsequently, IV maintenance therapy continued with 80% (0.08 μg/min/kg) of the standard recommended dosage (i.e., 0.1 μg/min/kg) used in myocardial infarction patients, for a maximum of 24 hours. With this method, we hoped to achieve focal and systemic elevation of tirofiban, good efficacy and fewer hemorrhagic complications. In our studies, 19 patients (95%) had their artery recanalized to TICI grade 3 from grade 0-2, with no related hemorrhagic complications. Five patients (25%) developed a thromboemboli-related cerebral infarction, 1 of whom was symptomatic.
In our study, 5 patients with ruptured cerebral aneurysm required mechanical devices to assist in thrombectomy. In 4 patients (including the embolic case), a microcatheter and microwire was used, and in 1 patient, a Solitaire stent was used. Of 3 patients who underwent thrombectomy assisted by microcatheter and microwire, preprocedure TICI grade 0 improved to TICI grade 2a after recanalization in 1 patient (who experienced severe vasospasm) and to TICI grade 3 in the other 2 patients. The fourth patient assisted by microcatheter and microwire had a distal embolism from carotid plaque that was TICI grade 1, but after recanalization of the vessel, the vessel improved to TICI grade 2a. In the patient who underwent thrombectomy with a Solitaire stent, the stent was originally intended to protect against coil protrusion, but it had to be retrieved because of a growing thrombus in spite of IA/IV tirofiban infusion and IV maintenance therapy lasting approximately 20 minutes. This patient’s vessel was recanalized, improving from TICI grade 1 to 3. Correspondingly, in 3 out of 5 patients with preprocedure TICI grade 0, and 2 out of 3 patients with preprocedure TICI grade 1, mechanical devices were used and the outcomes were relatively positive. Therefore, mechanical device-assisted thrombectomy could be a good adjunct option for dissolving thrombi during endovascular coil embolization, especially in patients with lower TICI grades. We decided to use mechanical devices within 20 minutes following tirofiban infusion, after obtaining serial angiograms at approximately 5-minute intervals because we believed that thrombus-dissolving action of tirofiban could be noted within several minutes. Thus, during the approximately 20-minute period, if the thrombus showed no changes or enlarged, we decided to use the mechanical devices for thrombectomy.
The limitations of this study were its retrospective analysis design and its small patient population. In addition, we could not explain the exact mechanisms of simultaneous IA and IV administration of tirofiban that were advantageous. However, in line with our results, simultaneous IA and IV loading and maintenance therapy appears to be a viable option for dissolving thrombi during endovascular coil embolization.
CONCLUSION
Thromboembolic events during endovascular coil embolization of cerebral aneurysm have been a challenge to neurointerventionists. Many pharmacological agents have been introduced to treat thromboembolism, and the outcomes appear promising. We found that in patients who develop thromboembolism during endovascular coil embolization of ruptured or unruptured cerebral aneurysms, administering a simultaneous IA and IV loading dose of tirofiban, followed by IV maintenance therapy, is a promising rescue treatment with good recanalization rates and low rates of bleeding complications.
Acknowledgements
This subject was presented in the 33rd Annual Spring Meeting of the Korean Neurosurgical Society on April 15th, 2015.
Fig. 1
Endovascular coil embolization was performed in a patient with a ruptured left intracerebral artery-anterior choroidal artery aneurysm. The left anterior choroidal artery (black arrows) was highly visible in the working view (A). After completing the coiling, the left anterior choroidal artery was still visible (B). In a follow-up angiogram taken 15 minutes later, the left anterior choroidal artery was not visible (C). Intra-arterial (via the guiding catheter)/intravenous loading was done at the same time with a dose of 5 μg/kg respectively (~80% of the usual recommended dose); tirofiban was maintained intravenously with a dose of 0.08 μg/kg/min (~80% of the usual recommended dose). Thirty minutes later, the flow through the left anterior choroidal artery was noted well (D), and intravenous maintenance therapy continued for approximately 23 hours. 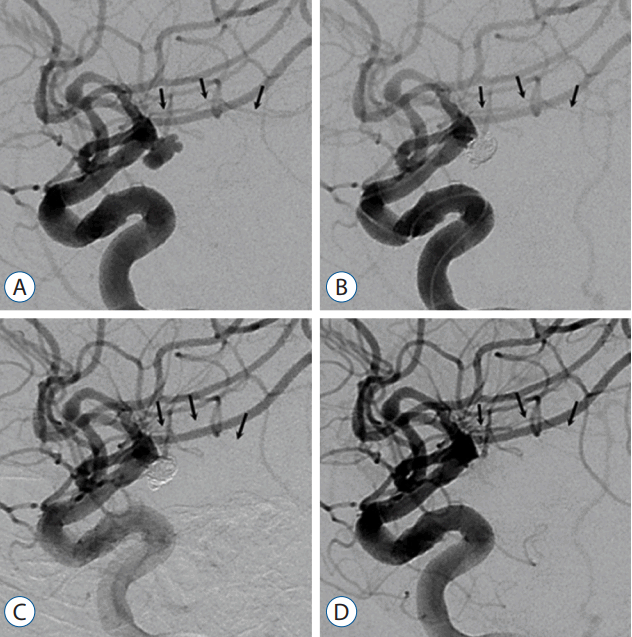
Fig. 2
Endovascular stent-assisted coil embolization was performed in a patient with a ruptured left anterior communicating artery aneurysm. The left anterior cerebral artery (A1 and A2; black arrows) was highly visible in the working view (A). After stent deployment and completion of coiling, the distal stent marker was noted at the left A2 (arrowhead) (B), and the acute in-stent thrombus and distal flow occlusion were noted in the left A1 (C). An intra-arterial (via the guiding catheter)/intravenous loading dose (5 μg/kg, ~80% of the usual recommended dose) was given at the same time, and tirofiban was maintained intravenously with a dose of 0.08 μg/kg/min (~80% of the usual recommended dose). Thirty minutes later, the flow of the left anterior cerebral artery was noted (D). After administering an oral loading dose of antiplatelets (aspirin 300 mg and clopidogrel 300 mg), intravenous maintenance therapy proceeded for 4 hours and was gradually discontinued. 
Table 1
Thrombolysis in cerebral infarction perfusion scale
|
Category |
Title |
Description |
|
Grade 0 |
No perfusion |
No antegrade flow beyond the point of occlusion. |
|
Grade 1 |
Penetration with minimal perfusion |
The contrast material passes beyond the area of obstruction but fails to opacify the entire cerebral bed distal to the obstruction. |
|
Grade 2 |
Partial perfusion |
The contrast material passes beyond the obstruction and opacifies the arterial bed distal to the obstruction. However, the rate of entry of contrast into the vessel distal to the obstruction and/or its rate of clearance from the distal bed are perceptibly slower than its entry into and/or clearance from comparable areas not perfused by the previously occluded vessel. |
|
Grade 2a |
|
Only partial filling (less than two-thirds) of the entire vascular territory is visualized. |
|
Grade 2b |
|
Complete filling of all of the expected vascular territory is visualized but the filling is slower than normal. |
|
Grade 3 |
Complete perfusion |
Antegrade flow into the bed distal to the obstruction occurs as promptly as into the obstruction and clearance of contrast material from the involved bed is as rapid as from an uninvolved other bed of the same vessel or the opposite cerebral artery. |
Table 2
|
Variable |
Value |
|
Gender |
|
Male |
12 (60) |
|
Female |
8 (40) |
|
|
Age (years) |
55.75±12.12 |
|
|
Aneurysm size (mm) |
|
Small (<5) |
7 (35) |
|
Medium (>5 and <10) |
12 (60) |
|
Large (>10) |
1 (5) |
|
|
Ruptured |
|
Ruptured |
14 (70) |
|
Unruptured |
6 (30) |
|
|
Aneurysm location |
|
Acom |
8 (40) |
|
MCA |
6 (30) |
|
ICA BBA |
2 (10) |
|
ICA-para |
1 (5) |
|
ICA-AntCho |
1 (5) |
|
IC-Pcom |
1 (5) |
|
A2-3 |
1 (5) |
|
|
Dome/neck ratio |
|
<1 |
3 (15) |
|
>1 and <2 |
13 (65) |
|
>2 |
4 (20) |
|
|
Treatment modality |
|
Single catheter |
9 (45) |
|
Double catheter |
1 (5) |
|
Balloon assisted |
1 (5) |
|
Stent assisted |
9 (45) |
|
|
Thrombus location |
|
Proximal |
4 (20) |
|
Neck |
15 (75) |
|
Distal |
1 (5) |
|
Embolus |
1 |
|
|
Thrombus origin |
|
The coil protrusion |
11 (55) |
|
In-stent |
4 (20) |
|
The coil protrusion with stent |
3 (15) |
|
Catheter displacement |
1 (5) |
|
The coil protrusion+emboi |
1* (5) |
Table 3
|
PreT/PostT |
0 |
1 |
2a |
2b |
3 |
|
0 |
|
|
1 |
|
4 |
|
1 |
|
|
1*
|
|
2 |
|
2a |
|
|
|
|
7 |
|
2b |
|
|
|
|
6 |
References
1. Bizzarri F, Scolletta S, Tucci E, Lucidi M, Davoli G, Toscano T, et al : Perioperative use of tirofiban hydrochloride (Aggrastat) does not increase surgical bleeding after emergency or urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg 122 : 1181-1185, 2001   2. Brinjikji W, McDonald JS, Kallmes DF, Cloft HJ : Rescue treatment of thromboembolic complications during endovascular treatment of cerebral aneurysms. Stroke 44 : 1343-1347, 2013   3. Brinjikji W, Morales-Valero SF, Murad MH, Cloft HJ, Kallmes DF : Rescue treatment of thromboembolic complications during endovascular treatment of cerebral aneurysms: a meta-analysis. AJNR Am J Neuroradiol 36 : 121-125, 2015    4. Bruening R, Mueller-Schunk S, Morhard D, Seelos KC, Brueckmann H, Schmid-Elsaesser R, et al : Intraprocedural thrombus formation during coil placement in ruptured intracranial aneurysms: treatment with systemic application of the glycoprotein IIb/IIIa antagonist tirofiban. AJNR Am J Neuroradiol 27 : 1326-1331, 2006   5. Cho YD, Lee JY, Seo JH, Kang HS, Kim JE, Jung KH, et al : Intra-arterial tirofiban infusion for thromboembolic complication during coil embolization of ruptured intracranial aneurysms. Eur J Radiol 81 : 2833-2838, 2012   6. Cognard C, Weill A, Castaings L, Rey A, Moret J : Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 206 : 499-510, 1998   7. Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J : Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol 19 : 157-165, 1998   8. Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F : Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 43 : 1281-1295; discussion 1296-1287, 1998   9. Eskridge JM, Song JK : Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg 89 : 81-86, 1998   10. Fiorella D, Albuquerque FC, Han P, McDougall CG : Strategies for the management of intraprocedural thromboembolic complications with abciximab (ReoPro). Neurosurgery 54 : 1089-1097; discussion 1097-1098, 2004    11. Fiorella D, Thiabolt L, Albuquerque FC, Deshmukh VR, McDougall CG, Rasmussen PA : Antiplatelet therapy in neuroendovascular therapeutics. Neurosurg Clin N Am 16 : 517-540, vi2005   12. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al : Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34 : e109-e137, 2003   13. Jeon JS, Sheen SH, Hwang G, Kang SH, Heo DH, Cho YJ : Intraarterial tirofiban thrombolysis for thromboembolisms during coil embolization for ruptured intracranial aneurysms. J Cerebrovasc Endovasc Neurosurg 14 : 5-10, 2012    14. Jeong HW, Jin SC : Intra-arterial infusion of a glycoprotein IIb/IIIa antagonist for the treatment of thromboembolism during coil embolization of intracranial aneurysm: a comparison of abciximab and tirofiban. AJNR Am J Neuroradiol 34 : 1621-1625, 2013    15. Kang HS, Kwon BJ, Roh HG, Yoon SW, Chang HW, Kim JE, et al : Intra-arterial tirofiban infusion for thromboembolism during endovascular treatment of intracranial aneurysms. Neurosurgery 63 : 230-237; discussion 237-238, 2008    16. Koebbe CJ, Horowitz MB, Levy EI, Dutton K, Jungries CC, Purdy PD : Intraarterial thrombolysis for thromboemboli associated with endovascular aneurysm coiling. Report of five cases Interv Neuroradiol 8 : 151-158, 2002   17. Marmur JD, Poludasu S, Agarwal A, Manjappa N, Cavusoglu E : High-dose tirofiban administered as bolus-only during percutaneous coronary intervention. J Invasive Cardiol 20 : 53-58, 2008  18. Mounayer C, Piotin M, Baldi S, Spelle L, Moret J : Intraarterial administration of abciximab for thromboembolic events occurring during aneurysm coil placement. AJNR Am J Neuroradiol 24 : 2039-2043, 2003   19. Park JH, Kim JE, Sheen SH, Jung CK, Kwon BJ, Kwon OK, et al : Intraarterial abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: outcome and fatal hemorrhagic complications. J Neurosurg 108 : 450-457, 2008   20. Pelz DM, Lownie SP, Fox AJ : Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 19 : 1541-1547, 1998   21. Ries T, Siemonsen S, Grzyska U, Zeumer H, Fiehler J : Abciximab is a safe rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization: single center experience in 42 cases and review of the literature. Stroke 40 : 1750-1757, 2009   22. Song JK, Niimi Y, Fernandez PM, Brisman JL, Buciuc R, Kupersmith MJ, et al : Thrombus formation during intracranial aneurysm coil placement: treatment with intra-arterial abciximab. AJNR Am J Neuroradiol 25 : 1147-1153, 2004   23. Velat GJ, Burry MV, Eskioglu E, Dettorre RR, Firment CS, Mericle RA : The use of abciximab in the treatment of acute cerebral thromboembolic events during neuroendovascular procedures. Surg Neurol 65 : 352-358; discussion 358-359, 2006   24. Viñuela F, Duckwiler G, Mawad M : Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 86 : 475-482, 1997   25. Workman MJ, Cloft HJ, Tong FC, Dion JE, Jensen ME, Marx WF, et al : Thrombus formation at the neck of cerebral aneurysms during treatment with Guglielmi detachable coils. AJNR Am J Neuroradiol 23 : 1568-1576, 2002  
|
|

















