Ki, Lee, Lee, Yi, Yang, and Lee: The Risk Factors for Hydrocephalus and Subdural Hygroma after Decompressive Craniectomy in Head Injured Patients
Abstract
Objective
The present study aims to investigate 1) the risk factors for hydrocephalus and subdural hygroma (SDG) occurring after decompressive craniectomy (DC), and 2) the association between the type of SDG and hydrocephalus.
Methods
We retrospectively reviewed the clinical and radiological features of 92 patients who underwent DC procedures after severe head injuries. The risk factors for developing post-traumatic hydrocephalus (PTH) and SDG were analyzed. Types of SDGs were classified according to location and their relationship with hydrocephalus was investigated.
Results
Ultimately, 26.09% (24/92) of these patients developed PTH. In the univariate analyses, hydrocephalus was statically associated with large bone flap diameter, large craniectomy area, bilateral craniectomy, intraventricular hemorrhage, contralateral or interhemisheric SDGs, and delayed cranioplasty. However, in the multivariate analysis, only large craniectomy area (adjusted OR=4.66; p=0.0239) and contralateral SDG (adjusted OR=6.62; p=0.0105) were significant independent risk factors for developing hydrocephalus after DC. The incidence of overall SDGs after DC was 55.43% (51/92). Subgroup analysis results were separated by SDG types. Statistically significant associations between hydrocephalus were found in multivariate analysis in the contralateral (adjusted OR=5.58; p=0.0074) and interhemispheric (adjusted OR=17.63; p=0.0113) types.
Conclusion
For patients who are subjected to DC following severe head trauma, hydrocephalus is associated with a large craniectomy area and contralateral SDG. For SDGs after DC that occur on the interhemispherical or controlateral side of the craniectomy, careful follow-up monitoring for the potential progression into hydrocephalus is needed.
Key Words: Decompressive craniectomy · Hydrocephalus · Subdural hygroma.
INTRODUCTION
Decompressive craniectomy (DC) has been widely used for the treatment of intractable intracranial hypertension for a prolonged period 2). The occurrence of hydrocephalus and subdural hygroma (SDG) after DC for severely injured trauma patients is relatively common 1,57,1314,1518,29). However, the mechanism by which these occur is still unclear. We have previously investigated the risk factors of hydrocephalus after DC for severely injured trauma patients in a small case series and found that delayed cranioplasty was statistically related to hydrocephalus 4). The aim of this study was to assess the incidence and risk factors for post-traumatic hydrocephalus (PTH) and SDG following DC with larger sample. An additional aspect of the study focused on the relationship between the type of SDG and hydrocephalus after DC.
MATERIALS AND METHODS
This retrospective study, by review of medical records, was performed on 191 patients who had received DC treatment at our hospital due to trauma-related acute intracranial hypertension from 2007 to 2014. The procedures for this study were approved by the Institutional Review Board of our hospital. Among the 191 patients, the present study was conducted on 92 participants, as they fulfilled the following inclusion criteria : they had survived beyond 3 months, follow-up observations were possible, and they had a minimum of four computerized tomography (CT) scan results. Unilateral DC involved wide removal of the skull, keeping a distance of approximately 3 cm from the midline, and passing over the frontoparietal cranium. Then, duraplasty was performed. Unilateral DC was performed on 83 patients, while 9 patients underwent bilateral DC procedures. Of the patients with bilateral DC, 6 patients received bilateral DC separately and 3 patients underwent a single procedure. The craniectomy distance from the midline was calculated by the post-operative skull antero-posterior (AP) view. We confirmed that skull AP radiography was magnified about 123% through the use of a real 3 cm marker at the vertex, which was verified by its comparison on the reconstructive 3D CT ( Fig. 1). The DC size was checked by the post-operative ipsilateral skull lateral view. We estimated the 2D area of the bone flap as follows : area=largest AP diameter (D)×diameter perpendicular to D (d)×π/4 8). Cases of traumatic subarachnoid hemorrhage were classified based on their thicknesses : ≥5 mm within the cistern perpendicular to the CT cross section or <5 mm. 11) Mid-line shifting was considered positive when 5 mm were excessed on the CT scan 17). The presence of SDG was noted if a hypodense mass with a thickness >5 mm was observed on the CT scan. When SDG was noted, it was further classified as ipsilateral, contralateral, or interhemispheric, based on where the craniectomy was performed ( Fig. 2). In bilateral DC cases, the contralateral type of SDG was not possible. Post-traumatic ventriculomegaly was defined as a frontal horn index ≥0.4 or modified frontal horn index ≥0.33 16,27). The determination of hydrocephalus was based on the following : indications of ventricular dilatation and transependymal edema on CT, indications of clinical deterioration or no improvement over time, and clinical improvements seen after ventriculoperitoneal shunting. During ventriculoperitoneal shunting, valves with pressure control devices were used. In addition to these factors, the initial Glasgow coma scale (GCS) score, time interval from craniectomy to cranioplasty, presence of intraventricular hemorrhaging, disappearance of basal cistern, traumatic intracerebral hemorrhage and subdural hemorrhage, and the cortical opening for hematoma removal were also analyzed. The amount of subdural hemorrhage was calculated the ABC/2 technique; as follows : A, the linear distance between each corner of the subdural crescent; B, the maximum thickness of the hematoma; and C, the product of the number of slices on which the hematoma was visible and the slice thickness on CT 10). All statistical analyses were performed using the software package SAS Enterprise Guide 5.1 (SAS Institute Inc., Cary, NC, USA) with a baseline p value <0.05 for statistical significance.
RESULTS
The baseline demographics and clinical characteristics of the 92 patients who had received DC are shown in Table 1. Patients included 68 males and 24 females with an average age of 52.84±17.20 years. The distribution of mild (GCS 9-15), moderate (GCS 6-8), and severe (GCS 3-5) impairment of the initial state were 43.48% (40/92), 30.43% (28/92), and 26.09% (24/92), respectively. Cases which needed arachnoid dissection or cortical opening for removal of hemorrhage during DC constituted 41.30% (38/92). Post-DC cranioplasty was performed in 84.78% (78/92), and the time interval between the procedures was 81.05±56.80 days. Cases with a subarachnoid hemorrhage thickness ≥5 mm were 16.30% (15/92) and cases accompanied by an intraventricular hemorrhage were 15.22% (14/92). Cases with acute subdural hemorrhage and intracerebral hemorrhage were 84.78% (78/92) and 29.35% (27/92), respectively. Disappearance of the basal cistern was seen in 58.70% (54/92), and 75% (69/92) showed shifting of the midline ≥5 mm. Cases of SDG were observed in 55.43% (51/92). Among those, ipsilateral, contralateral, and interhemispheric SDG were 46.74% (43/92), 16.30% (15/92), and 7.61% (7/92), respectively. Twelve patients have SDG in two or more locations. Ipsilateral and contralateral SDG was the most common pairing, accounting for 58% (7/12) of the double SDG instances.
Risk factors for post traumatic hydrocephalus after decompressive craniectomy
The incidence of post-DC hydrocephalus was 26.09% (24/92) and the mean age of the group with hydrocephalus was 53.25±18.26 years, occurring in 19 males and 5 females. The mean age for the group without hydrocephalus was 52.69±16.95 years, and included 49 males and 19 females. In hydrocephalus group, the mean largest AP diameter (D) of the bone flap was 146.81±16.97 mm, which is significantly statistically different than that of the non-hydrocephalus group, where D was 134.21±18.25 mm ( p=0.0012). The mean distance of the perpendicular diameter to D (d) in the hydrocephalus group was 98.26±6.02 mm, which is also statistically significantly larger than the non-hydrocephalus group's value of 91.76±8.76 mm ( p=0.0015). The large bone flap size was found to be closely associated with hydrocephalus ( p=0.0008) and an intraventricular hemorrhage was more frequently observed in the hydrocephalus group than in the non-hydrocephalus group ( p=0.0076). The bilateral craniectomy group exhibited a statistical relationship with hydrocephalus ( p=0.0468), and the removal of hematoma through the cortex was associated with hydrocephalus ( p=0.0488). The time between DC and cranioplasty in the hydrocephalic group was 113.81±74.27 days, whereas it was 68.98±43.76 days in the non-hydrocephalic group, which is statistically significantly different ( p=0.016). Other factors including gender, initial GCS score, craniectomy distance from mid-line, disappearance of the basal cistern, shifting of the midline and traumatic subarachnoid hemorrhage, subdural hemorrhage, and intracerebral hemorrhage did not show any statistically significant differences related to the occurrence of hydrocephalus. The overall SDG did not show relation to hydrocephalus. However, according to specific type of SDG, contralateral SDG showed hydrocephalus in 60.0% (9/15), but non-contralateral SDG or no-SDG showed hydrocephalus in 19.48% (15/77) ( p=0.0025). Interhemispheric SDG also showed hydrocephalus in 85.71% (6/7), but non-interhemispheric SDG or no-SDG showed hydrocephalus in 21.18% (18/85) ( p=0.0011) ( Table 1). In the univariate analyses, hydrocephalus was statically associated with the large bone flap diameter, large craniectomy area, bilateral craniectomy, intraventricular hemorrhage, contralateral or interhemisheric SDGs, and delayed cranioplasty. In the multivariate analysis, only the large craniectomy area (adjusted OR=4.66; p=0.0239) and contralateral SDG (adjusted OR=6.62; p=0.0105) were significant independent risk factors for developing hydrocephalus after DC ( Table 2).
Risk factors for subdural hygroma after decompressive craniectomy
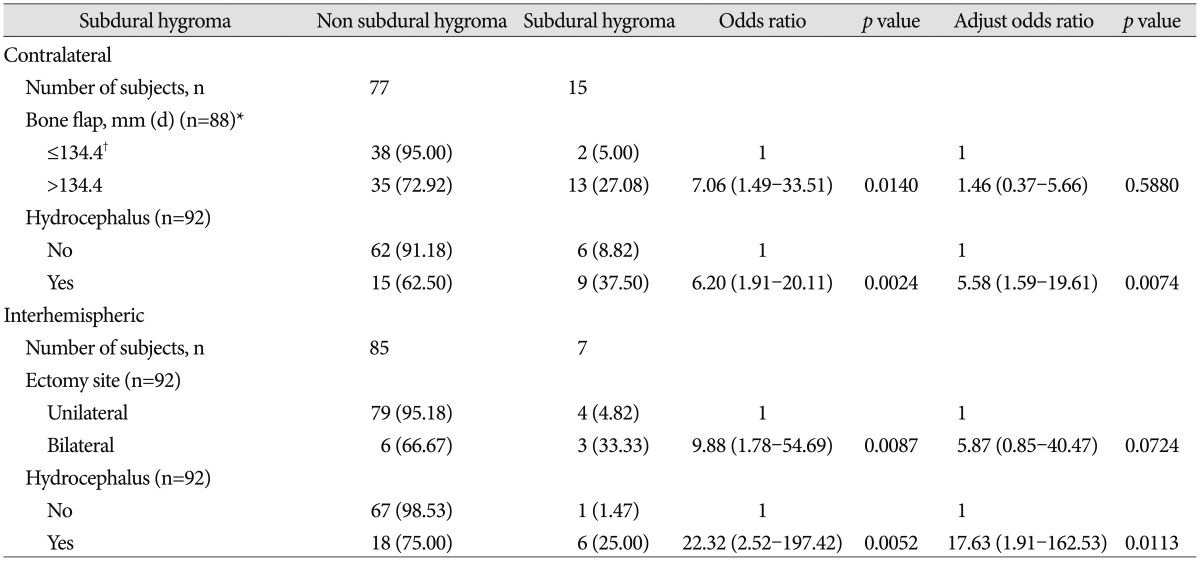
The incidence of overall SDG after a DC was 55.43% (51/92). All of the factors that were analyzed, including gender, initial GCS score, craniectomy distance from the midline, bone flap size, bilateral craniectomy, removal of hematoma through the cortex, disappearance of the basal cistern, shifting of the midline, traumatic subarachnoid hemorrhage, intraventricular hemorrhage, subdural hemorrhage, intracerebral hemorrhage, and cranioplasty interval did not show any statistically significant differences related to the occurrence of SDG. The subgroup analysis results were separated by SDG type. According to types of SDGs, all factors did not show any relation with the ipsilateral type. However, in the contralateral and interhemispheric type, only hydrocephalus showed statistical significance in the multivariate analysis (OR=5.58, p=0.0074; OR=17.63, p=0.0113) ( Table 3).
DISCUSSION
The incidence of PTH is reported as 0.7-29%, which varies widely based on the criteria by which hydrocephalus is determined 3,21). Reports have indicated that hydrocephalus may be result of circulatory disturbances and the malabsorption of cerebrospinal fluid (CSF) during the process of absorbing a hemorrhage from trauma, such as a spontaneous subarachnoid or intraventricular hemorrhage 9). Although Rahme et al. 24) reported that DC is not an independent risk factor for PTH, many patients that underwent a DC showed an increased incidence rate of hydrocephalus, about 9.3-40% 1,512,1518,2529). The reasons for this increased incidence of PTH in patients who received DC include trauma-related factors, in which patients that needed the DC might have suffered more severe injuries. Additionally, DC independently may induce circulatory disturbances of CSF and contribute to the development of hydrocephalus 5,712,1828,29). In the present study, the post-DC hydrocephalus incidence rate was 26.09% (24/92), and among the risk factors, large bone flap diameter, large DC area, bilateral craniectomy, intraventricular hemorrhage, delayed cranioplasty, and contralateral and interhemisperic SDG showed significant relationships with the development of hydrocephalus. This study is consistent with Choi et al. 5) result that the bilateral craniectomy may promote PTH. Through the multivariate analysis, we additionally found that only a large craniectomy area and contralateral SDG were significant independent risk factors for developing hydrocephalus after DC. Waziri et al. 28) found that DC causes the weakening of the systolic-diastolic pulsatile intracranial pressure difference, and ultimately leads to permanent malabsorption of CSF by the arachnoid. We believe that the large craniectomy area and delayed cranioplasty may result in the prominent and prolonged weakening of the systolic-diastolic pulsatile intracranial pressure difference, contributing to the hydrocephalus. Otherwise De Bonis et al. 7) described that the patients with craniotomy whose superior limit was <25 mm from the midline had an increased risk of developing hydrocephalus. If the DC margin is too close to the midline, this reduces the external force to the bridging vein and causes an increased venous outflow to the sinus. The increased extracellular fluid absorption results in the decreased volume of brain parenchyme and induces ventricular enlargement 7). However, this study did not find a correlation between the craniectomy margin from the midline and hydrocephalus. Age, gender, GCS score, subarachnoid hemorrhage, disappearance of the basal cistern, shifting of the midline, and the amount of hematoma showed no statistical differences regarding the occurrence of hydrocephalus. The incidence of traumatic SDG in patients not having had DC is known to be 4-22% 20,2223). After DC, the incidence is increased, and is reportedly approximately 26-63% 1,212,1415,1825,29). The occurrence of post-traumatic SDG is explained by a collection of CSF in the potential space between the dura mater and arachnoid by one-way valve action after traumatic injury 20). The arachnoid membrane usually adheres densely to the sphenoid wing so arachnoid tearing is likely to occur at the Sylvian fissure or chiasmatic region 6,22). Hemispheric SDG can develop in cases where the arachnoid tears at the Sylvian fissure and interhemispheric SDG may results from a chiasmatic lesion. Reasons for the increase in SDG after DC are explained by a number of factors. First, DC is performed mostly on more severely injured patients so the traumatic rupture of dura-arachnoid interface and trabeculae occurs often than in the non-DC patients. Second, the DC itself causes additional injury to the dura-arachnoid interface and increases the propensity of SDG. Kilincer et al. 19) reported a contralateral SDG after DC in a non-trauma-related patient. A large DC may cause a pressure gradient between the two hemispheres and contribute to the widening of the subdural space, resulting a SDG on the contralateral or interhemisperic side 18,19). Finally, the ipsilateral temporary collection of CSF occurs passively in expanded collection spaces when a duroplasty is performed in conjunction with DC 1,1225,29). Regardless of those reasons, most SDG after DC disappear without much clinical significance 1,225). However, in some cases, as the SDG gradually subsides, hydrocephalus slowly progresses 1,212,1518). In severe trauma patients, the mechanisms by which post-DC hydrocephalus and SDG occur are somewhat similar; they are thought to occur via the influence of both the initial trauma and the additional CSF circulatory disturbance after DC. Therefore, a correlation between the incidence rates of the two complications and risk factors may exist. 18) Jeon et al. 17) found that delayed hydrocephalus was more commonly seen in a SDG group (52.6%), but was only 10.4% in the group without SDG after DC. Su et al. 26) reported that patients with contralateral SDG after DC should be kept under close surveillance due to the risk of developing PTH. However, Aarabi et al. 1) reported that there was no correlation between post-DC SDG and hydrocephalus. In the present study, SDG was observed in 55.43% (51/92) of DC cases, with the majority of SDG of the simple ipsilateral type (62.75%, 32/51), and it disappeared over time. However, according to type of SDGs, 71% (5/7) of interhemispheric SDG and 60% (9/15) of contralateral SDG progressed into hydrocephalus. Unlike ipsilateral SDG that occurs in the space expanded simply by the craniectomy and duraplasty, interhemispheric and contralateral SDGs may closely associate with a CSF-circulatory disturbance. The present study showed a statistically significant relationship between interhemispheric and contralateral SDGs and the occurrence of PTH after DC. In the situation of CSF-circulatory disturbance and the abrupt pressure difference between both hemispheres after DC, in addition to the arachnoid tearing which had already occurred, it could be easier to make interhemisheric or contralateral SDGs than ventriculomegaly during the early period. At this time, brain tissue stiffness due to the brain injury and an edema may be the reason for the transient SDGs rather than a ventriculomegaly. As time goes by, these localized interhemisheric or contralateral SDGs can be easily converted to hydrocephalus. This study has limitations, due to its retrospective nature and the small number of patients that were enrolled. Therefore, a prospective study with a large sample is needed to draw firm conclusions.
CONCLUSION
In patients subjected to a DC following a severe head trauma, hydrocephalus is associated with a large craniectomy area and contralateral SDG. For the types of SDG after DC that occur on the interhemispherical or contralateral side of the craniectomy, careful follow-up monitoring is necessary for potential progression into hydrocephalus.
References
1. Aarabi B, Chesler D, Maulucci C, Blacklock T, Alexander M : Dynamics of subdural hygroma following decompressive craniectomy : a comparative study. Neurosurg Focus 2009, 26 : E8,  2. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM : Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg 2006, 104 : 469-479,   3. Cardoso ER, Galbraith S : Posttraumatic hydrocephalus--a retrospective review. Surg Neurol 1985, 23 : 261-264,   4. Cho BR, Lee HJ, Lee HJ, Yi JS, Yang JH, Lee IW : Risk factors for the post-traumatic hydrocephalus following decompressive craniectomy in severe traumatic injury patients. Korean J Neurotrauma 2012, 8 : 110-114,  5. Choi I, Park HK, Chang JC, Cho SJ, Choi SK, Byun BJ : Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. J Korean Neurosurg Soc 2008, 43 : 227-231,    6. Da Costa DG, Adson AW : Subdural hygroma. Arch Surg 1941, 43 : 559-567,  7. De Bonis P, Pompucci A, Mangiola A, Rigante L, Anile C : Post-traumatic hydrocephalus after decompressive craniectomy : an underestimated risk factor. J Neurotrauma 2010, 27 : 1965-1970,   8. Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C : Risk factors of aseptic bone resorption : a study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg 2013, 118 : 1141-1147,   9. Foltz EL, Ward AA Jr : Communicating hydrocephalus from subarachnoid bleeding. J Neurosurg 1956, 13 : 546-566,   10. Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, et al : Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke 1998, 29 : 1799-1801,   11. Greene KA, Marciano FF, Johnson BA, Jacobowitz R, Spetzler RF, Harrington TR : Impact of traumatic subarachnoid hemorrhage on outcome in nonpenetrating head injury. Part I : a proposed computerized tomography grading scale. J Neurosurg 1995, 83 : 445-452,   12. Honeybul S : Complications of decompressive craniectomy for head injury. J Clin Neurosci 2010, 17 : 430-435,   13. Honeybul S, Ho KM : Decompressive craniectomy for severe traumatic brain injury : the relationship between surgical complications and the prediction of an unfavourable outcome. Injury 2014, 45 : 1332-1339,   14. Honeybul S, Ho KM : Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J Neurotrauma 2012, 29 : 1872-1878,   15. Honeybul S, Ho KM : Long-term complications of decompressive craniectomy for head injury. J Neurotrauma 2011, 28 : 929-935,   16. Huh PW, Yoo DS, Cho KS, Park CK, Kang SG, Park YS, et al : Diagnostic method for differentiating external hydrocephalus from simple subdural hygroma. J Neurosurg 2006, 105 : 65-70,  17. Jeon SW, Choi JH, Jang TW, Moon SM, Hwang HS, Jeong JH : Risk factors associated with subdural hygroma after decompressive craniectomy in patients with traumatic brain injury : a comparative study. J Korean Neurosurg Soc 2011, 49 : 355-358,    18. Kaen A, Jimenez-Roldan L, Alday R, Gomez PA, Lagares A, Alén JF, et al : Interhemispheric hygroma after decompressive craniectomy : does it predict posttraumatic hydrocephalus? J Neurosurg 2010, 113 : 1287-1293,   19. Kilincer C, Simsek O, Hamamcioglu MK, Hicdonmez T, Cobanoglu S : Contralateral subdural effusion after aneurysm surgery and decompressive craniectomy : case report and review of the literature. Clin Neurol Neurosurg 2005, 107 : 412-416,   20. Lee KS : The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Inj 1998, 12 : 595-603,   21. Marmarou A, Foda MA, Bandoh K, Yoshihara M, Yamamoto T, Tsuji O, et al : Posttraumatic ventriculomegaly : hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J Neurosurg 1996, 85 : 1026-1035,   22. Murata K : Chronic subdural hematoma may be preceded by persistent traumatic subdural effusion. Neurol Med Chir (Tokyo) 1993, 33 : 691-696,   23. Ohno K, Suzuki R, Masaoka H, Matsushima Y, Inaba Y, Monma S : Chronic subdural haematoma preceded by persistent traumatic subdural fluid collection. J Neurol Neurosurg Psychiatry 1987, 50 : 1694-1697,    24. Rahme R, Weil AG, Sabbagh M, Moumdjian R, Bouthillier A, Bojanowski MW : Decompressive craniectomy is not an independent risk factor for communicating hydrocephalus in patients with increased intracranial pressure. Neurosurgery 2010, 67 : 675-678, discussion 678   25. Stiver SI : Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus 2009, 26 : E7,  26. Su TM, Lee TH, Huang YH, Su FW, Chen WF : Contralateral subdural effusion after decompressive craniectomy in patients with severe traumatic brain injury : clinical features and outcome. J Trauma 2011, 71 : 833-837,   27. Vanneste J, Augustijn P, Davies GA, Dirven C, Tan WF : Normal-pressure hydrocephalus. Is cisternography still useful in selecting patients for a shunt? Arch Neurol 1992, 49 : 366-370,   28. Waziri A, Fusco D, Mayer SA, McKhann GM 2nd, Connolly ES Jr : Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery 2007, 61 : 489-493, discussion 493-494   29. Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, et al : Surgical complications secondary to decompressive craniectomy in patients with a head injury : a series of 108 consecutive cases. Acta Neurochir (Wien) 2008, 150 : 1241-1247, discussion 1248  
Fig. 1
Skull AP (A), lateral (B), and 3D CT reconstruction (C : from above, D : from lateral) with real 3 cm marker after decompressive craniectomy. Distance from midline of skull AP (i) is 123% larger than the true distance on the 3D CT (I), as shown through a compared with a real 3 cm marker (white arrow). Largest AP diameter (D), distance perpendicular to D (d). AP : antero-posterior.
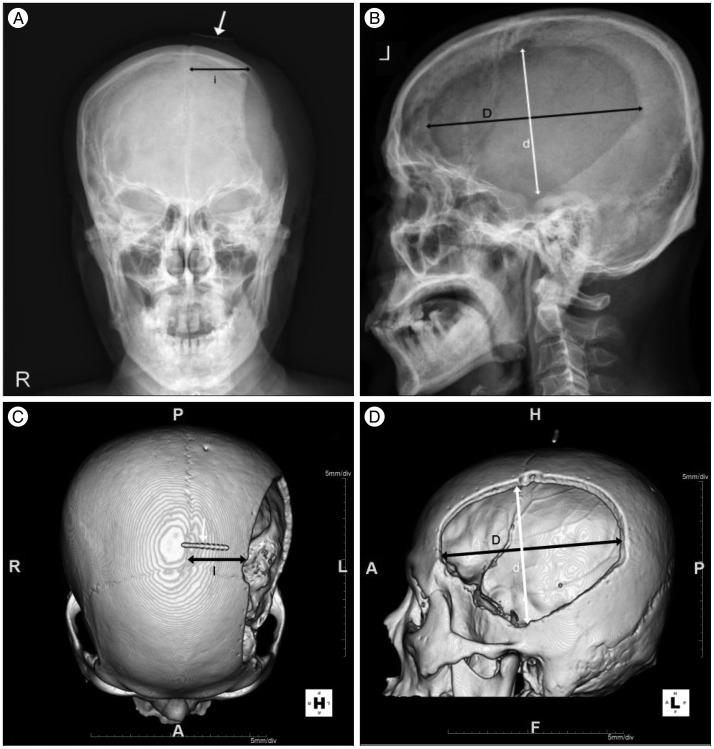
Fig. 2
Computerized tomography (CT) findings of a patient after decompressive craniectomy. A : CT at 12 days after decompressive craniectomy showing contralateral and ipsilateral subdural hygroma. B : CT at 55 days after decompressive craniectomy showed venetriculomagaly and a ventriculo-peritoneal shunt was placed after. C : CT at 28 days after decompressive craniectomy showing interhemispheric subdural hygroma. D : Patient required a ventriculo-peritoneal shunt due to hydrocephalus.
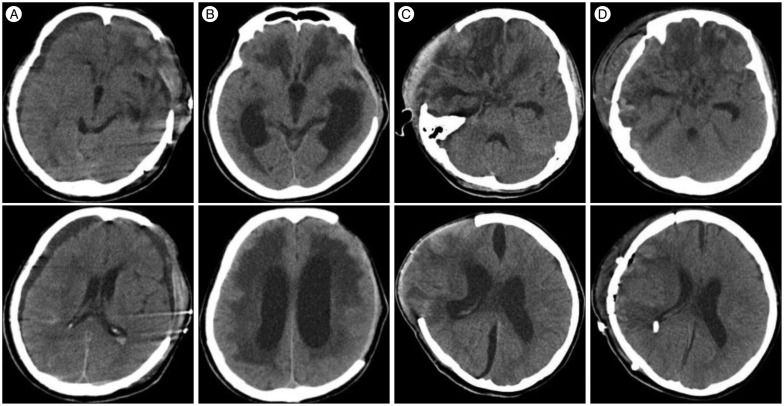
Table 1
Summary of characteristics in 92 patients without or with hydrocephalus following decompressive craniectomy for severe head injury
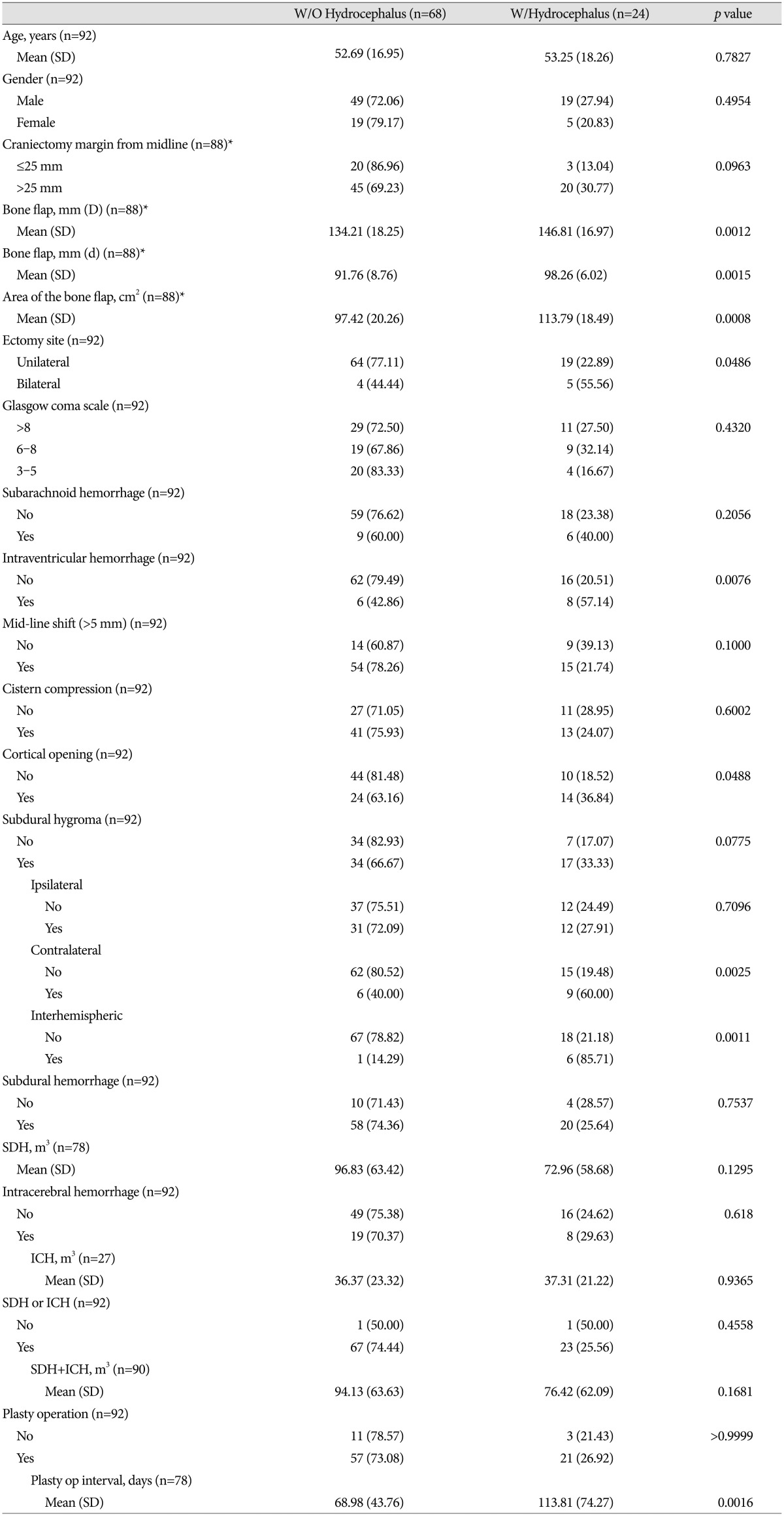
Table 2
Univariable and multivariable logistic regression analyses (prediction of hydrocephalus)
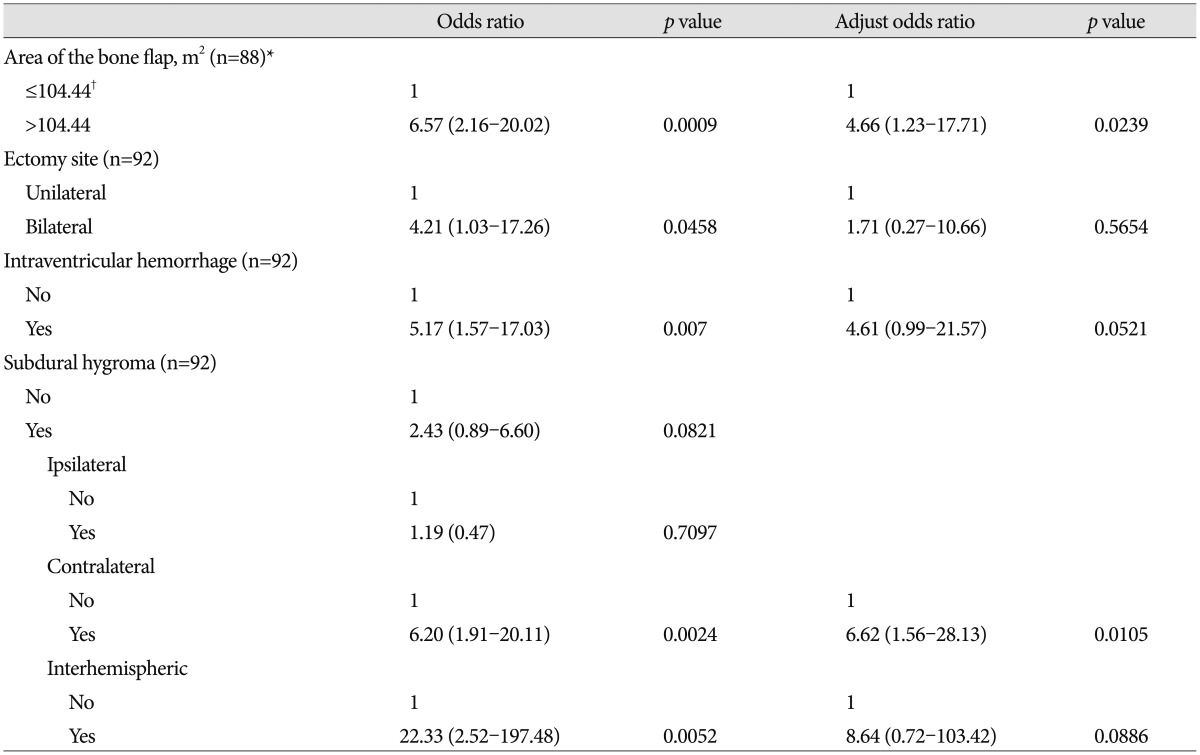
Table 3
Subgroup analysis stratified by subdural hygroma (contralateral type, interhemispheric type)
|
|




















