INTRODUCTION
Aneurysm clipping surgery is a conventional method to prevent further growth and rupture of an intracranial aneurysm4). The pterional approach is commonly used for clipping surgery, especially for an anterior circulation aneurysm24). During this approach, frontal lobe retraction is almost inevitable, especially in treating an anterior communicating artery (A-com) aneurysm. Frontal lobe retraction can cause detachment of the olfactory nerve from the cribriform plate, which causes olfactory dysfunction5). Postoperative olfactory dysfunction following surgical clipping for A-com aneurysm has been reported in 10-15% of patients2). Considering that olfactory function influences the quality of life and food intake13), this rate of olfactory dysfunction is too high to accept without reconsidering the pterional approach for A-com aneurysm surgery2).
Risk factors and the prevalence of postoperative olfactory dysfunction have been studied in some literatures. Old age (>55), a contra-lateral pterional approach, aneurysm size (>1 cm), location (especially within 3 cm from midline like A-com aneurysm), and ruptured aneurysm are associated with the development of postoperative olfactory dysfunction15,16). Until now, most studies which investigated postoperative olfactory dysfunction analyzed the incidence and prevalence of olfactory dysfunction in ruptured aneurysm treatment. The relationship between surgical treatment of an unruptured aneurysm and postoperative olfactory dysfunction has not been fully investigated, with only a few published studies addressing how to reduce the risk2).
Here we introduce a simple and safe technique to reduce postoperative olfactory dysfunction based on our experience.
MATERIALS AND METHODS
This study retrospectively included 69 patients who underwent surgical clipping for unruptured A-com aneurysm between January 2011 and September 2013. All procedures were performed by the same senior attending physician. Six patients were excluded because of unavailable data on preoperative olfactory function. The study retrospectively reviewed baseline characteristics of the aneurysm patients including gender, age, multiplicity of the aneurysm, side (ipsi- or contra-lateral from dominant A1) of surgical approach, size and projection of the aneurysm, and height from the anterior clinoid process. Multiplicity of the aneurysm was defined as treatment of two or more aneurysms in a single session (single craniotomy). We categorized projection of the aneurysm superior direction and others. Height of aneurysm was measured using preoperative sagittal computed tomography or magnetic resonance imaging and was categorized as more than 10 mm and below. Height was not measured in one patient due to unavailable any sagittal image. Distance was measured from clinoid process to neck of the aneurysm. Olfactory function was assessed with a simple olfactory function test involving recognition of the scent of coffee, tobacco, or hand cream preoperatively and immediately after surgery (within 3 days). Then, we checked for delayed olfactory function after more than 5 months later. We collected the results of the latest olfactory function test to determine the patient's olfactory function. Anosmia was defined as the complete loss of a subjective sense of smell-impossible identification of scent. Hyposmia was defined as a decreased sense of smell-possible identification of scent but patient complained of a subjective decline in the ability to smell. Olfactory dysfunction was defined as patients complaining of anosmia and/or hyposmia. Aneurysm size was measured based using three-dimensional (3D) angiography and other information (surgical approach, gross olfactory nerve injury, complications other than olfactory function, and past medical history) was collected from the medical record.
Surgical procedure and olfactory function evaluation
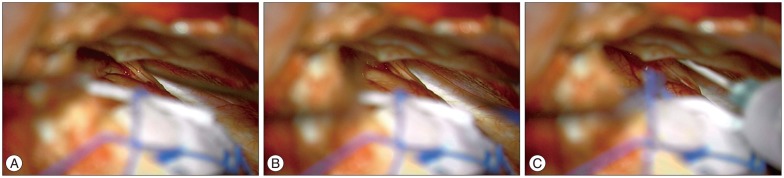
The surgical procedure was performed under the lateral supraorbital approach6). After dissecting the chiasmatic cistern and part of medial sylvian, the frontal base and olfactory nerve were divided. Thereafter, to prevent olfactory nerve detachment from cribriform plate, the gelfoam was embedded between the olfactory nerve and frontal lobe. Then, the regional area was sealed using fibrin glue (Fig. 1, 2). This simple olfactory protection method has been used since March 2012. The simple olfactory function test described above was performed during both the pre- and postoperative periods.
Statistical analyses
Statistical analyses were done using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Univariable statistical analysis (χ2 test or Fisher exact test) was performed to assess the associations between clinical radiological and procedural variables and olfactory dysfunction, which was defined as a categorical variable. The variables with p values<0.10 in univariable analysis were chosen for multivariable models by using multiple logistic regression analysis to evaluate the association factor with olfactory dysfunction.
RESULTS
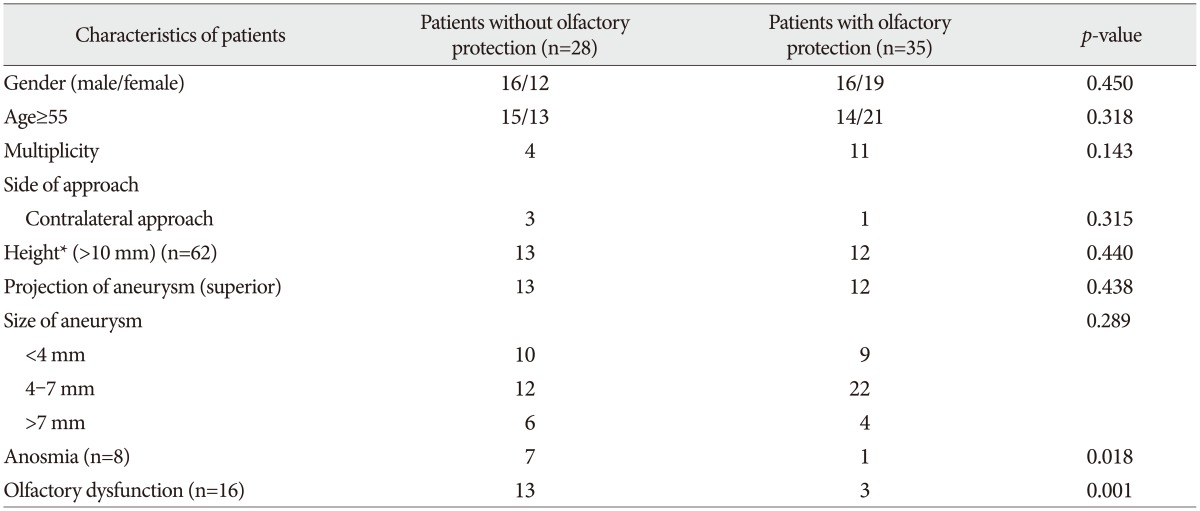
Sixty three patients (31 males, 32 females) were included in the study. Their median age was 54 years (range 35-72 years). The size of the aneurysm exceeded 7 mm in 10 patients, was 4-7 mm in 34 patients, and was smaller than 4 mm in the remainder. And 15 patients underwent surgical clipping in a single session for multiple aneurysms including A-com aneurysm. All patients underwent the clipping operation through the pterional approach, with ipsilateral approach for 59 patients and contralateral approach for 4 patients. Twenty five patients had superior projection A-com aneurysm. Thirty five patients had olfactory protection procedure during clipping surgery. During the operation, the olfactory nerve was detached from cribriform plate in three patients. Detachment upon identification of the olfactory nerve occurred in one patient even though steps were taken to protect the olfactory nerve. This patient was included in the protection group. There were no significant differences between protected group and unprotected group in age, sex, multiplicity, side, and projection and size of the aneurysm (Table 1). Sixteen patients developed postoperative olfactory dysfunction. Of these, eight patients were anosmia patients (protected group : unprotected group=1 : 7) and eight patients were hyposmia patients (protected group : unprotected group=2 : 6).
Univariate analysis revealed that superior projection aneurysm (p=0.016) and olfactory protection (p=0.001) were associated with postoperative olfactory dysfunction. Olfactory protection showed lower rate of postoperative anosmia (p=0.018) but superior projection of aneurysm tended to associate with higher incidence of anosmia but did not show statistically significant difference (p=0.052). Multivariate analysis using logistic regression testing showed that omission of olfactory protection was associated with high risk of anosmia [p=0.037, odds ratio (OR) 10.516, 95% confidence interval (CI) 1.159-95.449] and olfactory dysfunction (p=0.003, OR 9.676, 95% CI 2.176-43.014). Superior projection of the aneurysm showed higher olfactory dysfunction (p=0.015, OR 5.535, 95% CI 1.390-22.039) but did not show a statistically significant difference in anosmia (p=0.068, OR 5.227, 95% CI 0.886-30.819).
DISCUSSION
Ordinarily, the average risk of subarachnoid hemorrhage due to intracranial aneurysm rupture is 1% annually, and variable factors such as location, size, and morphology affect this rupture risk3,910,23). Therefore physicians consider these factors and other factors including age, past medical history, life expectancy, performance status, and patient's wishes in deciding whether an aneurysm should be treated and, if so, how to treat11). The two treatment options for unruptured aneurysms are craniotomy aneurysm clipping and endovascular treatment. Which one is better is debatable11,2022).
There are more risks related to surgical clipping compared to endovascular treatment, but one benefit is the reduced possibility of recurrence11,14). This is the basis for A-com aneurysm as a surgical strategy. A-com aneurysm clipping can be done through the pterional or interhemispheric approach7,18). Surgeons decide which approach to use after considering the aneurysm's location, projection, and relation to adjacent vessels19). The pterional approach is most commonly used in A-com aneurysm clipping by virtue of its various applications and surgeon familiarity1,16).
Potential complications from the clipping operation are altered mentality, weakness in the extremities, aphasia, mild headache, surgical scar, and olfactory dysfunction12). Complications rated less than 2 on the modified Rankin Scale are considered minor complications. As a result, olfactory function has generally been neglected, with only a few studies addressing the incidence of olfactory dysfunction after the pterional approach. A 39% incidence rate of anosmia was reported by Wermer et al.21) and 15% incidence of olfactory dysfunction including anosmia and hyposmia was reported by Aydin et al.2) and Cardali et al.5). Presently, the incidence of olfactory dysfunction in the unprotected group was 46%. This is higher than previous reports likely reflects the use of the lateral supraorbital approach, which needs more frontal lobe retraction than the routine pterional approach17).
Olfactory dysfunction can cause a lot of discomfort and inconvenience, especially associated with tasting and food ingestion13). During the pterional approach, surgeons need to retract the frontal lobe to visualize the aneurysm. Olfactory injury might be associated with length of retraction. Our results support this idea; aneurysms with superior projection were associated with greater risk of olfactory dysfunction. Retraction can increase the incidence of olfactory nerve detachment from the cribriform plate2), hence the concern about olfactory dysfunction as a serious complication of A-com aneurysm clipping surgery.
Presently, olfactory function was tested more than 5 months after surgery to determine if the patient developed permanent olfactory dysfunction. Right after surgery, with the associated pain, discomfort due to intubation, nasal mucosa edema, and temporary edema or malfunction of the olfactory system, many patients complain about temporary olfactory dysfunction. This is usually not permanent and disappears in a couple of days8). Sixteen of the 63 patients displayed olfactory dysfunction 5 months after surgery. Age, sex, size and height of aneurysm, and multiplicity seemed to be not related to the development of olfactory dysfunction. But, considering the relationship between frontal lobe retraction, aneurysm size and height, and multiplicity, studies involving more patients may be needed to confirm the relationship between these parameters and olfactory dysfunction2,5).
Olfactory protection and aneurysm projection have been significantly associated with postoperative olfactory dysfunction. Presently, postoperative olfactory dysfunction occurred less often in the group of patients who had olfactory protection during their surgery (p=0.001). The olfactory nerve detached from the cribriform plate before it was identified in one patient who was intended to receive olfactory protection. Even though we included this patient in the protection group, olfactory protection was concluded to preserve olfactory function during surgical clipping through the pterional approach.
The procedure for olfactory protection takes a couple of minutes, but it is not time consuming compared to the duration of the entire surgery. And this procedure is a simple procedure, and does not require special techniques. Also according to our investigation, there was no significant complication associated with this procedure.
Even though some techniques provide a favorable outcome for patients, they may not be suitable in situations where special material that is hard to get and expensive is required. But, the presently described procedure only requires gelfoam and fibrin glue, which are commonly used to control bleeding during surgery.
According to our results, olfactory protection technique is an acceptable method for preventing olfactory dysfunction during surgical clipping of an A-com aneurysm. The case where one patient in whom the olfactory nerve detached before it was identified highlights the limitation of the technique for other locations that do not need prominent frontal lobe retraction. So, our suggestion regarding this technique is restricted to the A-com aneurysm clipping operation, especially superiorly projected aneurysms. Further studies investigating relationships between aneurysm projection and postoperative olfactory dysfunction in protected patients are warranted to provide evidence supporting adoption of this protection procedure. Although our study did not show statistical significance between aneurysm height and olfactory dysfunction, a high location can be an another indication of olfactory protection in theory2,5).
Some limitations should be noted in this study. If a surgeon is concerned about protecting olfactory function in patients, this can cause selection bias-he or she will be more gentle and careful during the surgical procedure. In our study, to minimize other technical differences, all surgical procedures were performed by one senior attending physician.
The olfactory function test was simple and could be subjective. Future prospective studies with a larger cohort will better measure olfactory function through a psychophysiological test such as the University of Pennsylvania Smell Identification Test or an electrophysiological test like the olfactory event-related potential.
CONCLUSION
Postoperative olfactory dysfunction is a complication of A-com aneurysm surgery, which can reduce the quality of life. Our results suggest that superior projection of A-com aneurysm seems to be associated with postoperative olfactory dysfunction. And the simple olfactory protection technique might reduce the risk of postoperative olfactory dysfunction without any increase in complications, costs, and operation time. Prospective studies with a larger sample size are needed to further validate the usefulness of this technique.