INTRODUCTION
Meningiomas are the second most common central nervous system neoplasm in adults and account for 15-20% of all primary brain tumors. Although most meningiomas are benign, -10% of meningiomas demonstrate more aggressive clinical behavior, as non-benign meningiomas.
The term "chordoid meningiomas" was first coined by Kepes et al. as a histopathological classification of tumors removed in seven patients (aged 8-19 years) with Castleman's syndrome12). In the 2007 revision of the WHO classification of tumors of the CNS, chordoid meningioma (CM) joined clear cell meningioma and atypical meningioma in the grade II category, due to its high rate of recurrence, particularly following subtotal resection13).
Because of its rarity, there has been limited information about CM. There have also been some differences between studies about its aggressiveness and histopathological findings. Most previous studies have described the pathological features of CM precisely, but have lacked data on its clinical course or treatment outcomes. Furthermore, one recent study suggested that CM should be downgraded because of its relatively benign course25).
The purpose of this study was to verify the characteristics of CM. Thus, we analyzed the clinical features and treatment outcomes of 16 patients with CM and reviewed the literature.
MATERIALS AND METHODS
Patients
We performed surgical removal for 2599 cases of initially diagnosed intracranial meningioma in our hospital between January 1999 and December 2012. We reviewed the pathology data of these patients and 16 patients with pathologically confirmed CM were identified. All cases were reevaluated to confirm the histopathological diagnoses and to classify the tumors according to the 2007 WHO classification. We included all 16 patients in this study and retrospectively evaluated their medical records, radiological findings, and pathological findings.
Imaging studies, pathological review, radiation therapy, and follow up
All patients underwent preoperative computed tomography (CT) scans and magnetic resonance imaging (MRI). Tumor diameter was measured as the longest diameter of the tumor on enhanced T1WI. The extent of peritumoral edema was obtained from T2WI. CT scans were reviewed to check for bony invasion, defined as the existence of a focal osteolytic area adjacent to the tumor.
The extent of resection was determined by the operation records and postoperative MRIs, and was graded according to Simpson's classification22). Complete resection was defined as a Simpson Grade I resection. Incomplete resections were defined as Simpson Grade II, III, or IV resections.
In our treatment strategy, the first post-treatment MRI examination was done 3-6 months after the end of treatment and on an annual basis thereafter. Regardless of clinical symptoms, tumor recurrence or progression were defined as any newly detected enhancement after total resection or as any increased area of the residual tumor after subtotal resection, respectively. All documented cases of local recurrence were diagnosed radiographically and follow-up periods were measured from the time of the primary surgery.
To evaluate the proliferative capacity of individual tumors, the degree of Ki-67 labeling was determined using MIB-1 immunostaining in selected sections of excised specimens. Paraffin wax-embedded tissue sections were immunostained with an anti-MIB-1 antibody. The ratio of the MIB-1-positive cells to the total cell number was defined as the MIB-1 labeling index (%). There was no clear cut-off value that was defined as "high MIB-1 labeling index"; however, several previous studies have revealed that an MIB-1 labeling index score of >2-4% at initial surgery was associated with a high incidence of recurrence of meningiomas10,15,16,17,18,19). In the present study, a labeling index of Ōēź4% was deemed "high".
As there was no consensus on adjuvant radiation therapy for WHO grade II meningiomas, adjuvant radiation therapy was performed according to the individual physician's discretion for patients with incomplete resections. Patients with a complete resection were not considered as candidates for adjuvant radiation therapy. The median dose of radiation was 56.5 Gy (range : 54-60 Gy) in 27 to 35 fractions.
Progression-free survival (PFS) was determined on the basis of the length of time from the date of surgery to the appearance of radiological evidence of recurrence on follow-up MRI. Statistical analyses for PFS were performed by comparing computer-generated curves estimated by the Kaplan-Meier method. To evaluate the risk factors for recurrence, we used the log-rank test and Fisher's exact test. A p value <0.05 was considered to indicate statistical significance.
Follow-ups were conducted in the outpatient clinic, and the median follow-up period was 56.5 (range : 3-170) months. The median follow-up period of patients with recurrence was 55.5 (range : 3-170) months and patients with no recurrence was 56.5 (range : 14-89) months.
RESULTS
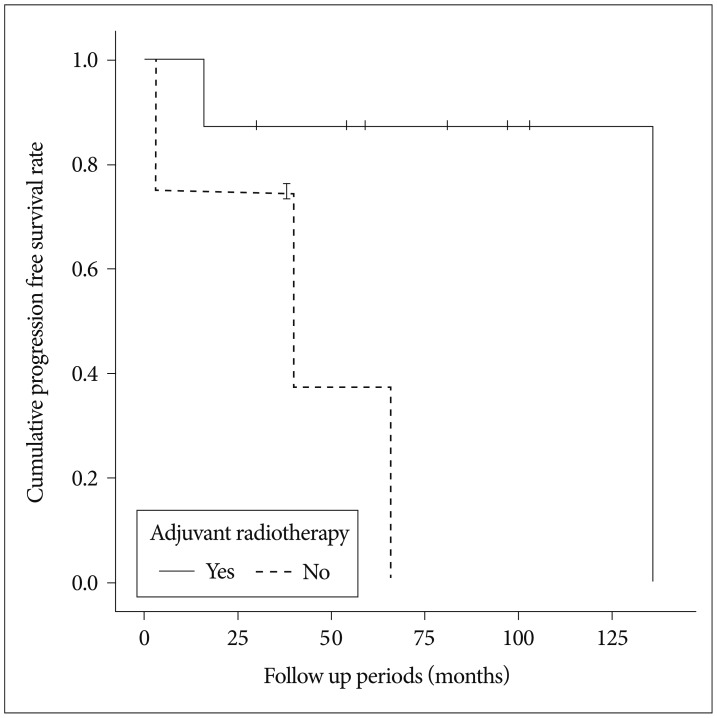
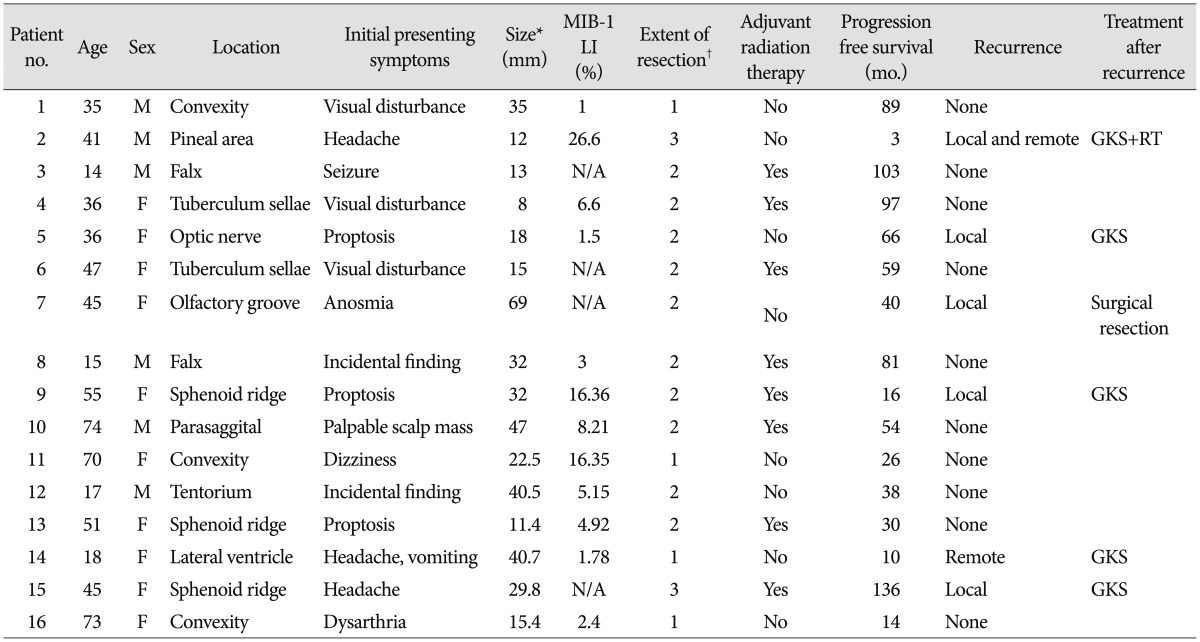
There were 6 men and 10 women (male-to-female ratio of 1 : 1.7). The mean age at the time of diagnosis was 40 (range, 14-74) years. The locations of tumors were as follows : convexity, 3; falx, 2; sphenoid ridge, 3; parasagittal, 1; intraventricular, 1; tuberculum sellae, 2; olfactory groove, 1; optic nerve sheath, 1; pineal area, 1; and tentorium, 1. Based on the MRI findings, preoperatively, three patients were suspected to have Langerhans cell histiocytosis (n=1), malignant glioma (n=1), and subependymal giant cell astrocytoma (n=1). Four patients showed bony invasion on CT scans. Of the 16 patients, 10 showed peritumoral edema to varying degrees. MIB-1 labeling index data were available for 12 patients and showed widely varying values (median : 5.04%, range : 1-26.6). A high MIB-1 labeling index (>4%) was seen in 7 of 12 (58.3%) specimens. One pediatric patient showed clinical features of Castleman's disease8) with a cerebellar CM and intestinal lymphangiectasia. Patient data, including initial symptoms and tumor size, are detailed in Table 1. Simpson grade I, II, and III resections were performed in four, nine, and three patients, respectively. No patient with a complete resection underwent adjuvant treatment. One of the 4 patients with Simpson grade I resection showed recurrence. In contrast, 5 of the 12 patients with incompletely resected tumors showed recurrence during the follow-up period (odds ratio, 2.14). Adjuvant radiation therapy was associated with longer PFS in the incompletely resected group. Among the 12 patients with incompletely resected tumors, eight underwent adjuvant radiation therapy. Patients who underwent surgery with adjuvant radiation therapy showed significantly longer PFS (121 months, 95% CI=82.1-159.9) than those who underwent surgery alone (40.5 months, 95% CI=9.6-71.3) by the log-rank test (p=0.027) (Fig. 1). Among the patients with recurrence (n=6), four showed local recurrence around the primary site, one showed distant intracranial recurrence, and one showed distant intracranial recurrence with spinal leptomeningeal seeding.
The average MIB-1 labeling index was 11.56% in cases with recurrence and 5.95% cases without recurrence. Focusing on the incompletely resected cases, it was 14.82% and 5.57%. However, it failed to reach statistical significance (Mann-Whitney U-test, p=0.731, p=0.451)
The treatments for recurring CM wereas as follows : Gamma knife radiosurgery (GKS), 4; GKS+radiation therapy, 1; and Endoscopic transnasal tumor resection, 1 (Table 1). One patient died after GKS and radiation therapy (Illustrated case). One patient showed tumor progression 11 months after GKS. Remaininr 4 patients showed no progression. The median follow-up period of these patients after recurrence was 23.5 months (range : 11-36).
In this series, we had two previous MRIs free of meningiomas, which were taken at 20 and 28 months before the CM diagnoses. In these patients, the estimated minimum growth rates were 1256 and 221 mm3/month (Fig. 2).
Illustrated case
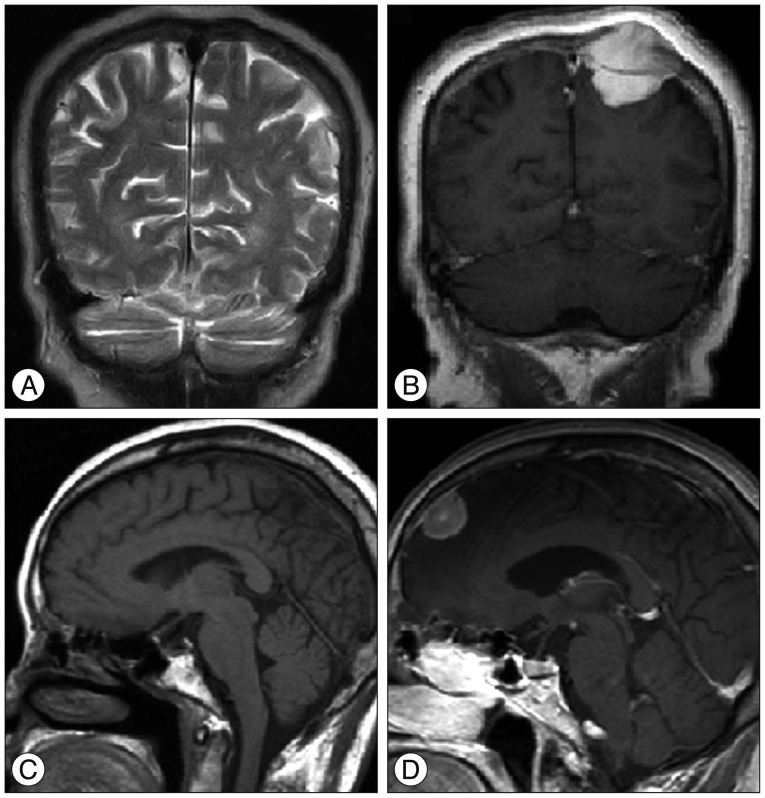
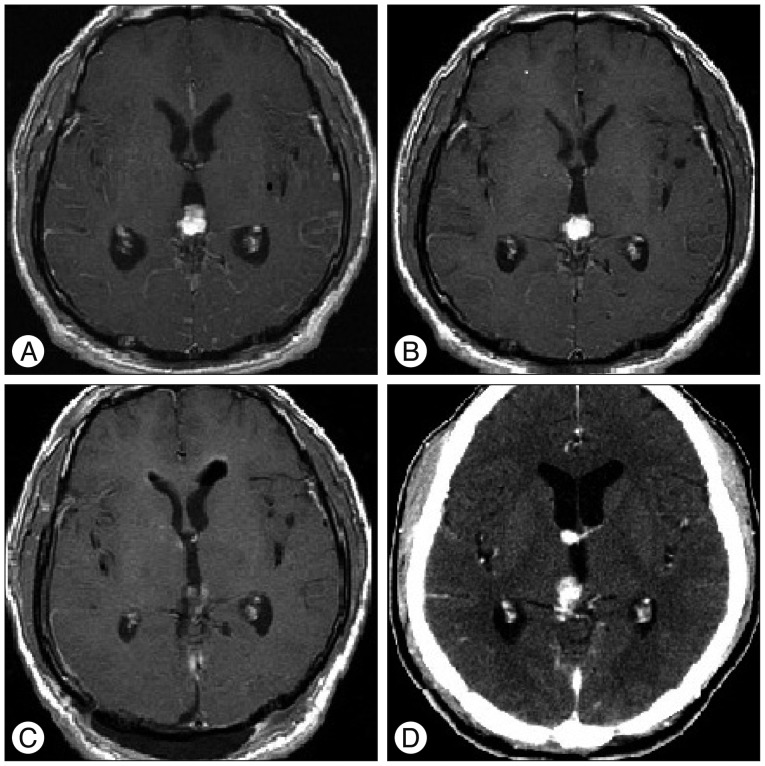
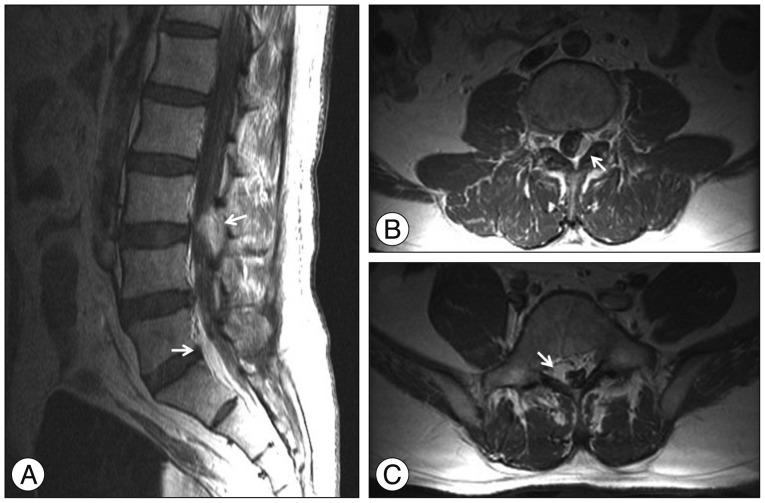
A 41-year-old male patient (Patient No. 2) (Table 1) visited the emergency department with severe headache and vomiting. The headache had developed a month ago and aggravated gradually. Axial T1-weighted contrast-enhanced MRI showed small enhancing mass at the pineal area with mild ventriculomegaly (Fig. 3A). The patient underwent endoscopic biopsy and third ventriculostomy (ETV), and CM was confirmed pathologically. An MRI was conducted 3 weeks after surgery and it showed slightly decreased ventricular size (Fig. 3B). Four weeks after surgery, we performed Simpson grade III resection with an occipitotranstentorial (OTT) approach. The post operative MRI showed no definite residual mass (Fig. 3C). CM was reconfirmed pathologically with an exceptionally high MIB-1 labeling index (26.60%). Three months after resection surgery, a contrast-enhanced CT was obtained for the follow up study, and it revealed the recurrencd of enhancing masses at the pineal area and right foramen of Monro (Fig. 3D). GKS (Rt. foramen of Monro : tumor volume 1100 mm3, prescription dose 25 Gy/50%, pineal area : tumor volume 322 mm3, prescription dose : 25 Gy/50%) was performed for recurred lesion and followed by radiation therapy (intensity-modulated radiation therapy, 40 Gy/20 fractions). Six months after resection surgery, intractable back pain developed, and contrast-enhanced spinal MRI showed multiple epidural enhancing masses at the thoracolumbar spine (Fig. 4). Radiation therapy was performed for spinal metastatic lesions. Fourteen months after resection surgery, the patient expired due to uncontrolled CNS metastasis of CM.
DISCUSSION
The first choice of treatment for CM is surgical excision. The ultimate goal of surgery is a complete resection to fully remove the tumor and the involved dura mater and skull.
However, the complete resection of a tumor is sometimes difficult because of its locatior. In this study, many CMs were located in the skull base or deep-seated in the brain, which would make their complete removal difficult. Because of the insufficient number of cases, it is difficult to verify the meaning of a complete resection statistically except for the odds ratio. Interestingly, the pattern of the resective procedure (i.e., en bloc vs. piecemeal resection) could be related with iatrogenic metastasis. Piecemeal resection was inevitable in a few cases depending on the location of the tumor particularly for intra-ventricle tumors, or those deep-seated in the brain. In fact, the primary locations of the CM were intra-ventricular and the pineal area in two cases of remote recurrence. Although there has been no previous literature about iatrogenic metastasis of WHO grade II meningiomas during surgery, few cases of iatrogenic metastasis during surgery have been reported. L├╝demann et al.14) reported a case of the iatrogenic seeding of malignant meningioma along a surgical trajectory, and Sadahira et al.20) reported on the implantation of malignant meningioma to the abdominal wall during resection surgery. The site of metastasis was the abdominal donor site of an autologous fat graft. Further clinical experience is required to clarify the importance of the pattern of the resective procedure in the case of WHO grade II meningiomas, including CM.
Radiation therapy in patients with meningiomas is controversial. However, clinical benefits have been reported, especially for the treatment of residual tumors, recurrence, and both WHO grade I and III meningiomas2,4,6,7). However, theres have been no randomized or prospective studies. We performed adjuvant radiation therapy fon eight patients with Simpson grade II and III resections, at the physician's discretion. Because of its rarity, in patients with CM, clinical experience with adjuvant radiation therapy has not yet been accumulated. Recently, Wang et al.25) reported on the benefits of adjuvant radiation therapy after incomplete CM resection. Our results support adjuvant radiation therapy in cases of incomplete resection. However, in cases of Simpson grade I resection, recurrence only occurred in only one of four patients and it was a remote-site recurrence. Based on this result, adjuvant radiation therapy after Simpson grade I resection for local tumor control may not always be recommended.
The MIB-1 labeling index increases with WHO grade : 1.00-1.35% for grade I, to 1.90-9.30% for grade II or atypical, and 5.60-19.5% for grade III1,3,5,21). Moreover, a higher MIB-1 labeling index is related to meningioma recurrence5,21,24,26). However, other studies have refuted these relationships between MIB-1 labeling index and the clinical features of meningiomas11,23,25). In this series, the average MIB-1 labeling index in cases with recurrence was higher than cases without recurrence, but failed to reach statistical significance. Focusing on incompletely resected cases, the gap of MIB-1 labeling index between the groups with recurrence and without recurrence widened, but it also failed to reach statistical significance. However, the only case with the development of leptomeningeal seeding after resection surgery was the case with the highest MIB-1 labeling index. Because of the small number of cases, it is hasty to conclude the clinical meaning of MIB-1 labeling index in this study. Accumulation of more clinical experience is demanded to so that this issue can be clarified.
The MIB-1 labeling index for CM in this series was quite variable, at 1.0-26.6%. This indicates that biological nature may be diverse even within the single pathological disease entity of CM. As a result, the MIB-1 labeling index may provide more information about biological features and the prognosis for each CM case.
Interestingly, two patients underwent brain MRIs at 20 and 28 months before diagnosis. We made inferences from the MRIs and the minimum growth rates in these two patients were faster than the previously reported growth rates for benign or incidentally found meningiomas9,27). However, this inference was based on only two cases of CM. It seems to be wise to avoid hasty generalizations.
Recently, some have suggested downgrading CM in the WHO classification unless poor prognostic factors are found. In our experience, this suggestion seems to result from the wide spectrum of biological characteristics within this single disease entity. However, regarding our series, with the high recurrence rate, especially in the non-adjuvant radiation therapy group, the possibility of leptomeningeal seeding, the relatively high growth rate, and high MIB-1 labeling index in some cases, it is difficult to argue that CM is a benign meningioma.
There are some limitations to our study; the number of patients was not sufficient to determine biological or clinical characteristics. Additionally, potential biases may exist, because of the collected clinical data being reviewed retrospectively. Because of the rarity of CM, a multicenter prospective randomized study is required to clarify the various controversies that have been noted previously.