INTRODUCTION
Remote cerebellar hemorrhage (RCH) is defined as bleeding in the cerebellum as a complication of surgery performed at a location distant from the site of bleeding. It is extremely rare after spinal surgery and has been reported more frequent after supratentorial surgery (0.2-4.9%)1,4). Although the precise mechanism of RCH after spinal surgery is unknown, intensive postoperative cerebrospinal fluid (CSF) loss is a well-documented causative factor8).
RCH usually extends to the folia and vermis close to the tentorium in the upper part of the cerebellum. It manifests on computed tomography (CT) as the "zebra sign," which refers to the high-attenuation curvilinear hemorrhage between the low-attenuation cerebellar folia5). Dozens of case reports concerning RCH after spinal surgery have been published, and bilateral cerebellar hemorrhages are a common feature. However, we recently experienced a case of multiple cerebral hemorrhage as well as cerebellar hemorrhage in a 63-year-old man who had large amount of CSF leakage after lumbar spinal surgery.
CASE REPORT
A 63-year-old man presented with a one-month history of bilateral buttock pain. He had past history of spinal surgery (posterior lumbar and interbody fusion at L3-4 and L4-5) before 1-year due to the same symptom. He had essential hypertension that was being controlled with a calcium channel blocker and an angiotensin converting enzyme inhibitor. Anticoagulants had not been administrated, and bleeding tendency was not noted before surgery. Recently, he had claudication after walking, and preoperative lumbosacral spine magnetic resonance image (MRI) revealed disc herniation at the level of L5-S1.
The patient's surgical treatment involved L5-S1 discectomy, L3-L5 laminectomies, and screw fixation of the L3-L5 pedicles. The dura mater was damaged intraoperatively due to adhesion caused by previous surgery and sutured in a water-tight fashion. There were no apparent complications such as perioperative hypertension or hypoxia. The patient awoke from anesthesia neurologically intact.
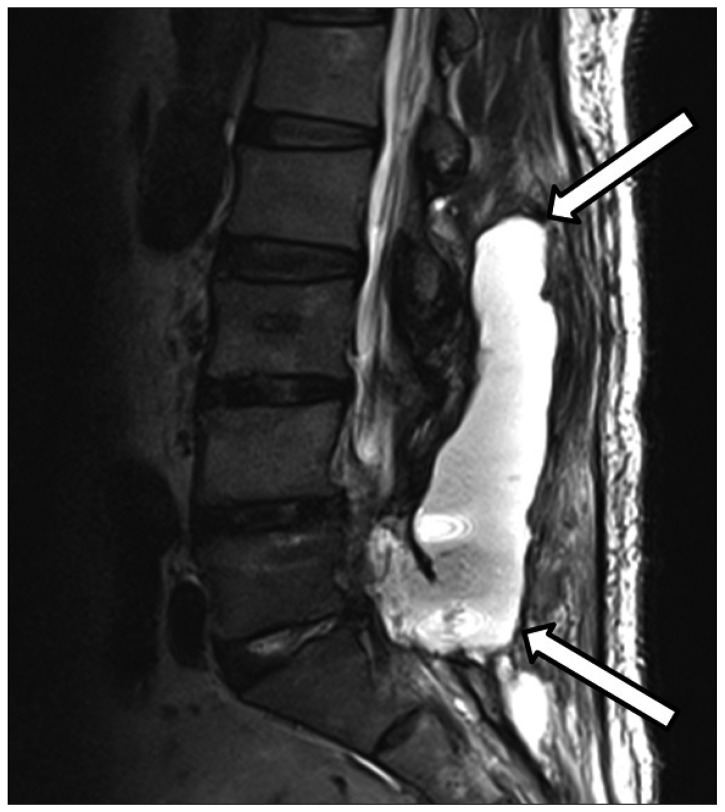
On the first postoperative day, the patient's percutaneous drainage tube drained 810 mL. Drainage was not noted the next day, so the drainage tube was removed on the second postoperative day. However, on the third postoperative day, CSF leakage was noted and the patient complained of back pain. A lumbosacral MRI obtained at the 4th postoperative day demonstrated a large amount of CSF leakage in the lumbosacral space (Fig. 1). At that time, the patient complained only of back pain without significant neurologic symptoms or postural headache. CSF leakage was conservatively managed but was persistent on a MRI obtained at the 8th postoperative day.
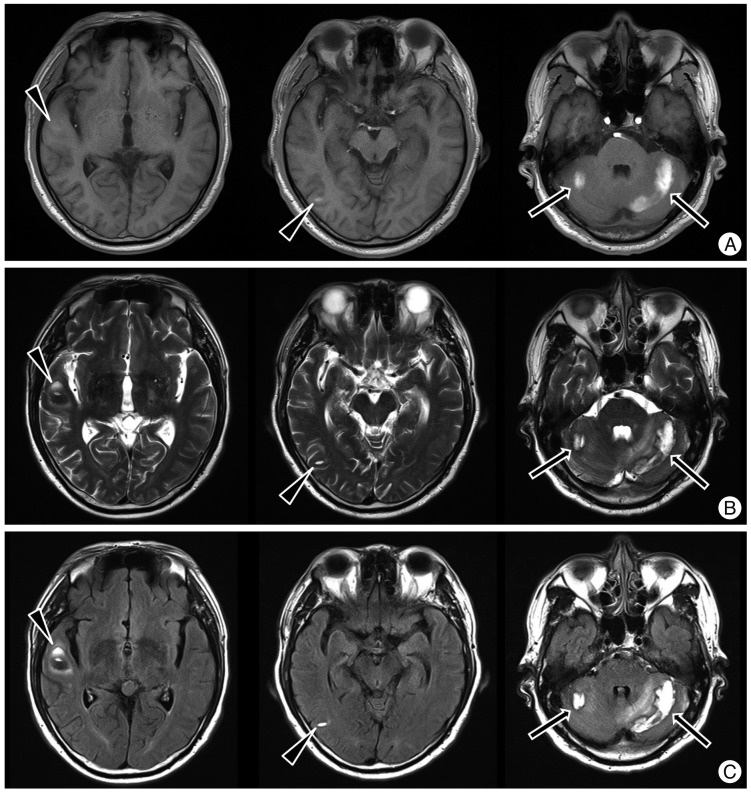
The patient underwent the second operation to repair the torn dura mater at the 10th postoperative day. The operation was performed in prone position and a dural defect was noted on L5 thecal sac. The dural defect was sutured in a water-tight fashion. During operation, a large amount of CSF was gushed out, but the amount of leaked CSF was not measured. The patient complained of headache upon awakening from anesthesia, and headache lasted for six days. A brain MRI was performed on the 16th postoperative day and showed acute and subacute hemorrhages in the bilateral cerebellar hemispheres and the right temporal lobe (Fig. 2). Brain CT showed hemorrhagic lesions in the same locations (Fig. 3). Correlating with clinical history, the imaging studies were compatible with multiple remote intracerebral and cerebellar hemorrhages. The patient was managed conservatively with anti-edema treatment, analgesics, and bed rest until the 25th postoperative day. The neurologic examination remained normal one year after surgery.
DISCUSSION
We experienced a rare case of massive lumbosacral CSF leakage resulting in multiple cerebral and cerebellar hemorrhages. Intracranial hemorrhage after craniotomy or spinal surgery is one of the most common and important complications in neurosurgery1). Previous reports have described several hemorrhages that occur at the surgical site, while others develop at remote locations1). Many authors have reported cases in which RCH developed as a delayed complication of spinal surgery8). RCH was first reported by König et al.9)in 1987. The authors found that cerebellar hemorrhage occurred in 0.3% of 1350 cases of supratentorial craniotomy performed between 1981 and 1983. After that report, several other cases of cerebellar hemorrhage after spinal surgery and supratentorial craniotomy were subsequently reported6,12,14). In the most of cases, the location of hemorrhage is cerebellum as its name, and it has propensity to locate bilaterally8). Involving cerebral parenchyma as well as cerebellum is even more rare, so just a few cases have been reported3,11,13). Most recently, Bowers et al.3) reported the case of a patient who developed cerebral and cerebellar intraparenchymal hemorrhages after spinal surgery.
Although the pathophysiological mechanism of cerebellar hemorrhage after spinal surgery is unknown, CSF leakage seems to be important in the pathogenesis of this complication. One proposed mechanism is an increase in the transluminal venous pressure caused by intracranial hypotension from CSF loss, resulting in blood vessel rupture10). Another possible mechanism is that downward cerebellar sag causes stretching and occlusion of the bridging cerebellar veins, with subsequent hemorrhagic venous infarction2). Although CSF leakage was persistent on MRI between the 4th and 8th postoperative days, the patient did not complain of headache prior to the dural repair surgery. The patient complained of headache upon awakening from anesthesia of the dural repair surgery, and acute and subacute hemorrhage was noted on MRI taken 6 days after the dural repair surgery. We think that hemorrhage developed during the dural repair surgery since a large amount of CSF was gushed out during the operation. Thus, we support the hypothesis proposed by Morandi et al.11) that movement of the brain due to intracranial hypotension from massive CSF loss can cause acute occlusion of multiple infra- and supratentorial bridging veins. This would explain the presence of multiple foci of cerebral and cerebellar hemorrhages.
Imaging findings for RCH usually consist of bilateral extra-axial bleeding in the subarachnoid space of the cerebellar hemispheres, resulting in a typical linear morphology parallel to the cerebellar folia. The alternating pattern of linear hyperdensities produced by the bleeding and the relatively hypodense cerebellar folia gives rise to the so-called zebra sign5). Intra-axial bleeding in the cerebellar hemispheres had been also frequently reported3,10). In our patient, zebra sign was not evident and intra-axial bleeding was noted in the temporal lobe and cerebellum. In our case, MRI and CT were taken 6 days after dural repair surgery. We believe that small amount of subacute subarachnoid hemorrhage could not be demonstrated on MRI and CT.
The most common symptoms of RCH are mental status change and headache. Other symptoms include motor deficits, gait ataxia, and seizure3,7). Unfortunately, the diagnosis may be delayed for several reasons. First, some patients remain asymptomatic. Second, it is difficult to evaluate cerebellar function after spinal surgery using gait testing. Third, postoperative brain imaging is not routinely performed if the patient is neurologically intact. Unfortunately, delayed diagnosis can result in larger or additional hemorrhages. In our patient, we did not consider cerebral or cerebellar hemorrhage as a cause of his headache because it is a rare complication of spinal surgery. The condition was finally diagnosed according to MRI obtained 6 days after he underwent dural repair surgery.
Treatment depends on the patient's clinical condition4). RCH appears to be a self-limiting phenomenon and should not be mistaken for more dangerous conditions such as hemorrhagic infarction. Most cases do not require further diagnostic evaluation or surgical intervention. However, surgical intervention may be indicated in hemorrhages large enough to cause a mass effect that leads to obstructive hydrocephalus. Follow-up brain imaging can be helpful to assess the progression of the hemorrhage.
Prognosis depends on the severity of the hemorrhage4). In general, patients with multiple hemorrhages have a poor prognosis. It is unclear why our patient's hemorrhages were extensive in the supratentorial location compared to those in other reported cases of RCH after spinal surgery. Fortunately, no significant neurologic deficits occurred in our patient.
CONCLUSION
It is important to consider the possibility of remote supratentorial or infratentorial hemorrhage in any patient with a large amount of CSF leakage after spinal surgery, even in the absence of significant neurologic symptoms. We recommend diagnostic imaging as soon as possible to ensure prompt diagnosis and treatment.