Jo, Im, Kong, Seol, Nam, and Lee: Gamma Knife Radiosurgery for Brain Metastases from Breast Cancer
Abstract
Objective
The authors conducted a retrospective cohort study to determine prognostic factors and treatment outcomes of brain metastases (BM) from breast cancer (BC) after Gamma Knife radiosurgery (GKS).
Methods
Pathologic and clinical features, and outcomes were analyzed in a cohort of 62 patients with BM from BC treated by GKS. The Kaplan-Meier method, the log-rank test, and Cox's proportional hazards model were used to assess prognostic factors.
Results
Median survival after GKS was 73.0 weeks (95% confidence interval, 46.0-100.1). HER2+ [hazard ratio (HR) 0.441; p=0.045], Karnofsky performance scale (KPS) вүҘ70 (RR 0.416; p=0.050) and systemic chemotherapy after GKS (RR 0.282; p=0.001) were found to be a favorable prognostic factor of overall survival. Actuarial local control (LC) rate were 89.5Вұ4.5% and 70.5Вұ6.9% at 6 and 12 months after GKS, respectively. No prognostic factors were found to affect LC rate. Uni- and multivariate analysis revealed that the distant control (DC) rate was higher in patients with; a small number (вүӨ3) of metastasis (HR 0.300; p=0.045), no known extracranial metastasis (p=0.013, log-rank test), or the HER2+ subtype (HR 0.267; p=0.027). Additional whole brain radiation therapy and metastasis volume were not found to be significantly associated with LC, DC, or overall survival.
Conclusion
The treatment outcomes of patients with newly diagnosed BM from BC treated with GKS could be affected primarily by intrinsic subtype, KPS, and systemic chemotherapy. Therapeutic strategy and prognosis scoring system should be individualized based on considerations of intrinsic subtype in addition to traditionally known parameters related to stereotactic radiosurgery.
Key Words: Brain metastasis В· Breast cancer В· Gamma Knife radiosurgery В· Intrinsic subtype В· Treatment outcomes.
INTRODUCTION
Brain metastasis (BM) is one of the most devastating complications of cancer and is usually associated with poor prognosis 2,10). Improvements in the systemic treatment of cancer and survival mean that the control of central nervous system (CNS) involvement has become increasingly important to achieve overall disease control 10). BM in breast cancer (BC) differs from BM from other primaries for several reasons. The first concerns the high incidences of BM and relapse in BC, which vary from 25 to 40% 3,19). The second is that BM in BC is more radiosensitive than BM from other primaries, such as, melanoma or renal cell carcinoma, and the third reason is that prognosis is better after BM diagnosis 4). For these reasons additional whole brain radiation therapy (WBRT) after local treatment such as stereotactic radiosurgery for BM from BChave been recommended for patients with a good prognosis 1,20). But there appears to be a lack of consensus regarding optimal treatment because of known adverse radiation effect. Furthermore, until comparatively recently, the majority of comparative studies conducted on treatment modalities failed to consider primary cancer intrinsic subtype, even after the development of targeted therapy. Accordingly, treatment outcomes should be re-investigated to determine the roles of treatment modalities and the effects of intrinsic subtype in BM from BC. In this study, we retrospectively reviewed a cohort of patient with BM from BC treated by Gamma Knife radiosurgery (GKS) and assessed the impacts of other treatment methods, clinical and pathological variables on the risks of brain relapse and overall survival.
MATERIALS AND METHODS
The study population
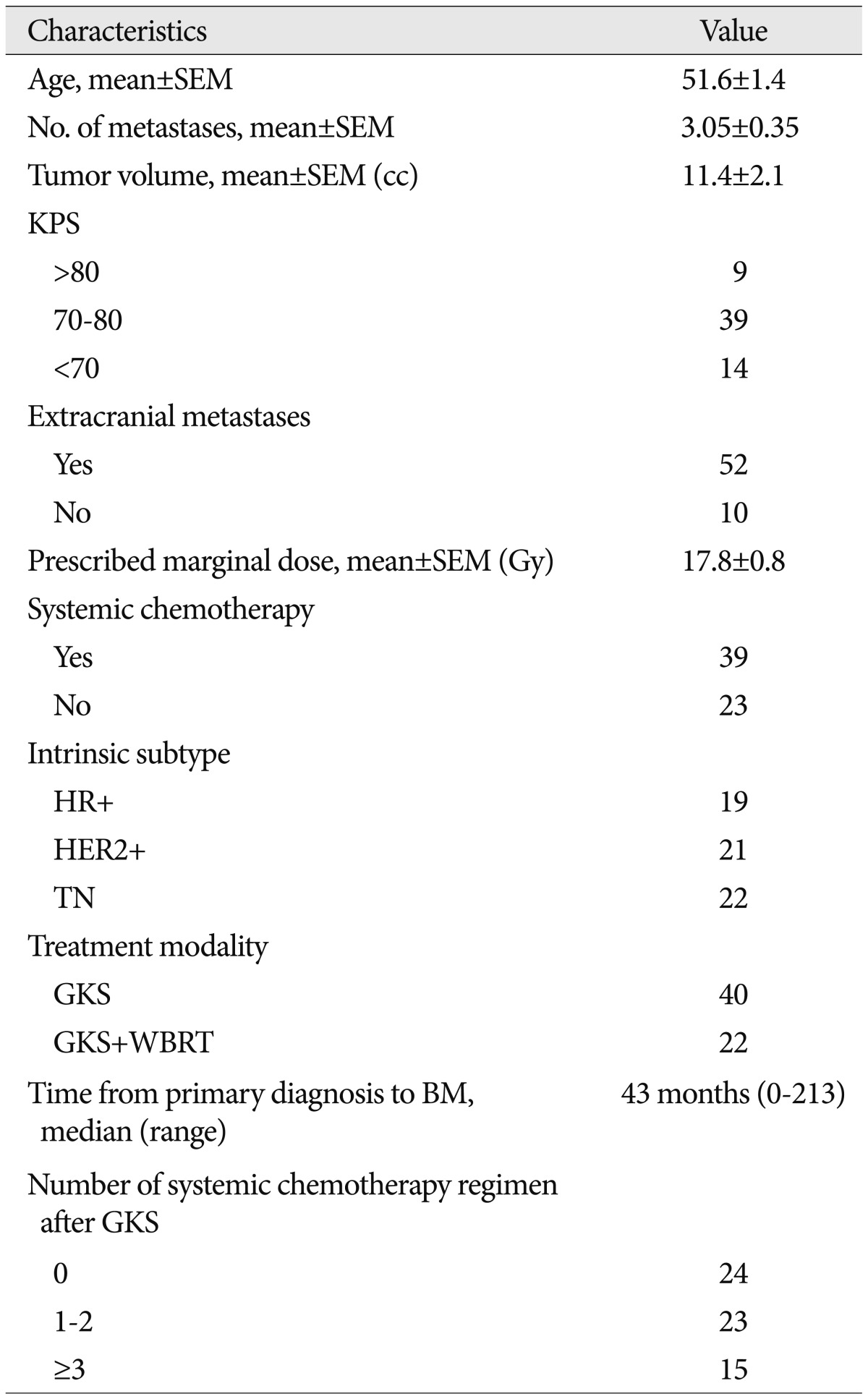
We identified 156 patients with BM from BC treated by GKS between 2002 and 2011 in our GKS Center database. Patients treated by GKS as a first line treatment were included, and all had confirmed adenocarcinoma of the breast. The exclusion criteria applied were; no assessable data on extracranial metastasis status, no assessable intrinsic subtype status, no clinical follow-up, and receipt of other treatment for BM before GKS, such as, surgery or WBRT. Of the 156 patients, 105 underwent GKS as initial treatment modality. Total of 62 fulfilled these eligibility criteria. Patient baseline characteristics are summarized in Table 1. We compared patients with respect to receptor status, as determined by immunohistochemical staining (IHC) [estrogen receptor (ER), progesterone receptor (PgR), and (HER2)]. ER and PgR positivity were defined as Allred scores of 3-8 by IHC using antibodies to ER (Immunotech, France) or PgR (Novocastra, UK). HER2 status was evaluated by antibody analysis (DAKO, CA, USA) and/or by fluorescence in situ hybridization (FISH). Grades 0 and 1 for HER2 by IHC were defined as negative results, and grade 3 as a positive result. Amplification of HER2 was confirmed by FISH if HER2 was rated 2+ by IHC. Triple negative (TN) was defined as the absence of ER, PgR, and HER2 by FISH. Of the 62 patients, 19 patients were HR+ (ER+ and/or PgR+ and HER2-), 21 were HER2+ (HER2+ regardless of ER and/or PgR), and 22 were TN (ER-, PgR-, and HER2-).
Follow-up protocol and data collection
Clinical evaluations were performed 1 month after treatments, and MRI was performed every 2 or 3 months thereafter. In patients with detected recurrence, further treatment was administered at the discretion of the attending physician.
Medical records and laboratory and imaging findings were reviewed, and the following were included; patient age, type of treatment for BM, Karnofsky performance scale (KPS) score, status of extracranial metastases, number and volume of metastases, administration of systemic chemotherapy, duration of follow-up, and radiologic evidence of local or distant control. Death or last follow-up were defined as study end points. Local control (LC) failure was defined as an increase in diameter of >25% of an initially GKS-treated lesion on contrast-enhanced MR images or the need for resection, and distant control (DC) failure was defined as the development any new brain metastatic lesion. Patients who had received any type of systemic chemotherapy for more than two cycles or two months were defined as having received systemic chemotherapy.
Statistical analysis
The chi-square test was used to compare group categorical data. For the primary analysis, overall survival time was calculated from GKS to death from any cause. Secondary analyses included LC and DC periods, which were calculated from the time of GKS treatment until LC or DC failure. The patients were stratified by clinical characteristics, and survivals were estimated across strata using the Kaplan and Meier method and compared using the log-rank test. Cox's proportional hazards model was used to estimate the relative risks for treatment modalities or intrinsic subtypes after adjustment for variables potentially associated with LC, DC, or overall survival. Data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was accepted for p values of <0.05.
RESULTS
In 105 patients who treated with GKS as an initial treatment, mean age at the time of GKS was 50.3 and 83 (78.3%) had KPS no less than 70. Mean tumor volume and number were 9.9 cc and 3.78 cc, respectively. Prescribed marginal dose showed 18.48 on average. Median overall survival was 72.0 weeks [95% confidence interval (CI), 54-89.975].
In the study population (n=62), the mean age at the time of GKS for BM was 51.6Вұ1.4 years, and 48 (77.4%) had KPS no less than 70. Mean tumor volume and number were 11.4 cc and 3.05 cc respectively. Prescribed marginal dose showed 17.8 Gy on average. These baseline characteristics did not differ from that of patients (number=105) who underwent GKS as initialtreatment. Other demographic and clinical characteristics of patients are summarized in Table 1. Forty-five of the 62 patients had died at the time of review. Median overall survival was 73.0 weeks (95% CI, 46.0-100.1). LC and DC failure occurred in 14 and 23 of the 62 patients, respectively. Overall survival, LC rate and DC rate were not different between GKS alone group from additional WBRT group. Six- and 12-month actuarial DC rates were 80.7% and 77.0% in GKS alone group and 93.7% and 67.5% in additional WBRT group, respectively ( p=0.181).
Treatment outcomes by intrinsic subtype
Summary of patient details according to intrinsic subtype described in Table 2. Median overall survivals were; 104.0 weeks (95% CI, 27.8-180.2) for HR+, 103.0 weeks (95% CI, 60.9-229.1) for HER2+, and 46.0 weeks (27.6-64.4) for TN, respectively ( p<0.05, log-rank test) ( Fig. 1). Multivariate analyses of overall survival showed HER2+ subtype was associated with longer survival ( Table 3). Overall 6- and 12-month actuarial rates of LC were; 87.5% and 79.5% for HR+, 88.2% and 61.7% for HER2+, and 87.7% and 81.5% for TN ( p=0.720, log-rank test). Overall 6 and 12-month actuarial rates of DC were 91.6% and 65.3% for HR+, 93.8% and 86.5% for HER2+, and 81.1% and 65.3% for TN ( p=0.327, log-rank test). Thus, univariate analysis showed that LC and DC rates were not different for intrinsic tumor subtypes. But multivariate analysis showed that HER2+ status was associated with the higher rate of DC ( Fig. 2, Table 5).
Factors associated with LC, DC, and overall survival
In 105 patients who treated GKS as initial treatment median overall survival was 73.0 weeks (95% CI, 55.7-90.3). Univariate analyses using log-rank test showed high KPS (p<0.001) and receipt systemic chemotherapy (p<0.001) were associated with favorable outcomes, while age (p=0.214), number (p=0.196) and volume (p=0.254) of metastases, status of extracranial metastases (p=0.085), and omission of WBRT (p=0.371). Since the results of whole population were beyond the scope, following results were presented focused on 62 study population.
Uni- and multivariate analyses of overall survival showed that a small number of metastases, a high KPS, and the receipt of systemic chemotherapy predicted longer survival ( Table 3). However, previously identified prognostic factors, age, number, and volume of metastases, status of extracranial metastases, and treatment modality were not significantly related to overall survival. Uni- and multivariate analyses failed to identify any factor significantly associated with LC rate ( Table 4). However, univariate analysis revealed that a small number of metastases (вүӨ3) and the absence of extracranial metastasis were associated with DC ( Table 5). In this study, additional WBRT did not showed statistical significance in LC, DC and overall survival.
DISCUSSION
This retrospective cohort study was conducted on breast cancer patients with BM treated at a single-institution from 2002 to 2011. Overall survival in the present study was longer than those reported previously 4,11,15), which could be due to a high proportion of younger patients (вүӨ65) and an overall good performance status (KPSвүҘ70). Furthermore, previously identified prognostic factors, such as, treatment modality and volume and number of brain metastases were not found to affect treatment outcomes significantly. Patients' homogeneity who was elegible to GKS might affect to these results. Nevertheless, we also attribute this result to a betterunderstanding of tumor biology, early diagnosis of brain metastases, and the adoption of multimodal treatments, which included targeted drug therapies, surgery, radiosurgery, and radiotherapy. Le Scodan et al. 9) examined outcomes for WBRT in BC patients with BM, and concluded that a low KPS, and tumor intrinsic subtypes were for significant prognostic value. Our results support these findings. However, in the present study, previously reported prognostic factors for brain metastasis, such as, number and volume of brain metastases, age, and additional WBRT were not found to be significantly associated with LC or overall survival. Only a large number of metastases were found to be significantly related to DC rate. These findings suggest that established scoring systems, such as, RPA, GPA, and SIR, cannot accurately predict outcomes of BM from BC 14). Because generally well known prognostic factors considered in decision of radiosurgery or radiotherapy do not take into account primary tumor subtype, and thus, many previous studies concerning radiosurgery seems to have been conducted using incomplete prognosis scoring systems 6-8,14). Accordingly, the progress made in tumor biology and targeted therapy means that cancer-specific prognostic systems are needed to better characterize patients and devise individualized treatment strategies 6). Aoyama 1) recently reviewed treatment outcomes of BM from BC, and concluded that WBRT is still important in this context of modern radiotherapy despite delayed adverse effects on cognition and functional independence 2,13). In our study, we found that additional WBRT did not influence LC, DC, or survival. Furthermore, actuarial curves showed that DC and overall survival rates in GKS alone vs. additional WBRT crossed at 37 and 55 weeks. These findings suggest the effect of WBRT on recurrence does not give significant influence in long term survivors. They caution that treatment scheme should be modified to meet the expectations of physicians and patients 12,18). In this study, LC rate was not affected by any prognostic value such as age, KPS, intrinsic subtype or WBRT status. Which may suggest that local recurrence did not affect prognosis after GKS for BM from BC. This result may reflect high LC probability of GKS. And better survival and distant control were found to be driven by the HER2+ subset. Previously, HER2+ overexpression indicates an aggressive subtype of BC characterized by rapid cell proliferation, increased angiogenesis, deficient apoptosis, and increased metastasis formation. Accordingly, HER2+ is considered a negative prognostic factor that affects new and recurrent brain metastases in BC 5). However, recent reports which showed treatment outcome regarding to intrinsic subtype suggest that patients with CNS metastases and HER2+ have better survival than patients with CNS metastases and HER2 10,16,17,20), which may be due to the differences in biological background and treatment improvements related to HER2-targeted therapy 10). These results suggest that other unknown genetic factors, rather than HER2+, affect outcome. Our data suggest that elevated observed rates of recurrent BM in HER2+ BC were due to prolonged survival, rather than any causative relation. Several limitations of the current study warrant consideration. First, this study included patients who were treated with GKS for brain metastases initially and whose tumor intrinsic subtype could be identified. The selection bias may have inflated median survival and deviated the results of analyses. But the main objective of our study was to analyze the effect of biological subtype on treatment outcomes, therefore we had to select the patients who could provide whole parameters of interest. Also, all 106 patients who underwent GKS as initial treatment showed similar baseline characteristics and similar results on univariate analyses results. Therefore, we thought this data might possess representativeness. Second, the effects of secondary treatments after LC and DC failure were not considered and third, the cohort size was small.
CONCLUSION
In this study, the clinical courses of patients with BM from BC were found to be affected by BC subtype, performance score, and systemic chemotherapy. These results might suggest that therapeutic strategy and prognosis scoring system should be individualized to reflect these factors. Additional data from large-scaled, randomized controlled trials are required to determine the role of additional WBRT and to devise a disease specific prognostic scale for BM from BC.
Acknowledgements
This study was supported by a grant from the Korean Healthcare technology R&D project, Ministry for Health & Welfare Affairs, Republic of Korea (grant no. A092255).
References
1. Aoyama H : Radiation therapy for brain metastases in breast cancer patients. Breast Cancer 2011, 18 : 244-251,   2. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al : Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation : a randomised controlled trial. Lancet Oncol 2009, 10 : 1037-1044,   3. Duchnowska R, Dziadziuszko R, Czartoryska-ArЕӮukowicz B, Radecka B, Szostakiewicz B, SosiЕ„ska-Mielcarek K, et al : Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat 2009, 117 : 297-303,   4. Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW : Survival in patients with brain metastases from breast cancer : the importance of HER-2 status. Cancer 2008, 112 : 2359-2367,   5. Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, et al : Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 2006, 24 : 5658-5663,   6. Golden DW, Lamborn KR, McDermott MW, Kunwar S, Wara WM, Nakamura JL, et al : Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases : variation by primary site. J Neurosurg 2008, 109( Suppl):77-86,  7. Goyal S, Prasad D, Harrell F Jr, Matsumoto J, Rich T, Steiner L : Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg 2005, 103 : 218-223,   8. Gu HW, Sohn MJ, Lee DJ, Lee HR, Lee CH, Whang CJ : Clinical analysis of novalis stereotactic radiosurgery for brain metastases. J Korean Neurosurg Soc 2009, 46 : 245-251,    9. Le Scodan R, Massard C, Mouret-Fourme E, Guinebretierre JM, Cohen-Solal C, De Lalande B, et al : Brain metastases from breast carcinoma : validation of the radiation therapy oncology group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys 2007, 69 : 839-845,   10. Lee S, Ahn HK, Park YH, Nam DH, Lee JI, Park W, et al : Leptomeningeal metastases from breast cancer : intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat 2011, 129 : 809-817,   11. Liu MT, Hsieh CY, Wang AY, Chang TH, Pi CP, Huang CC, et al : Prognostic factors affecting the outcome of brain metastases from breast cancer. Support Care Cancer 2006, 14 : 936-942,   12. Matsumoto K, Ando M, Yamauchi C, Egawa C, Hamamoto Y, Kataoka M, et al : Questionnaire survey of treatment choice for breast cancer patients with brain metastasis in Japan : results of a nationwide survey by the task force of the Japanese Breast Cancer Society. Jpn J Clin Oncol 2009, 39 : 22-26,   13. Monje ML, Palmer T : Radiation injury and neurogenesis. Curr Opin Neurol 2003, 16 : 129-134,   14. Nieder C, Marienhagen K, Astner ST, Molls M : Prognostic scores in brain metastases from breast cancer. BMC Cancer 2009, 9 : 105,     15. Ono M, Ando M, Yunokawa M, Nakano E, Yonemori K, Matsumoto K, et al : Brain metastases in patients who receive trastuzumab-containing chemotherapy for HER2-overexpressing metastatic breast cancer. Int J Clin Oncol 2009, 14 : 48-52,   16. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH : Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol 2009, 20 : 56-62,   17. Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, et al : Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 2009, 100 : 894-900,    18. Siu TL, Jeffree RL, Fuller JW : Current strategies in the surgical management of cerebral metastases : an evidence-based review. J Clin Neurosci 2011, 18 : 1429-1434,   19. Tsukada Y, Fouad A, Pickren JW, Lane WW : Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 1983, 52 : 2349-2354,   20. Wolstenholme V, Hawkins M, Ashley S, Tait D, Ross G : HER2 significance and treatment outcomes after radiotherapy for brain metastases in breast cancer patients. Breast 2008, 17 : 661-665,  
Fig.В 1
Accumulative survival rates according to KPS (p=0.000), systemic chemotherapy (p=0.000), and breast cancer intrinsic subtype (p=0.027). 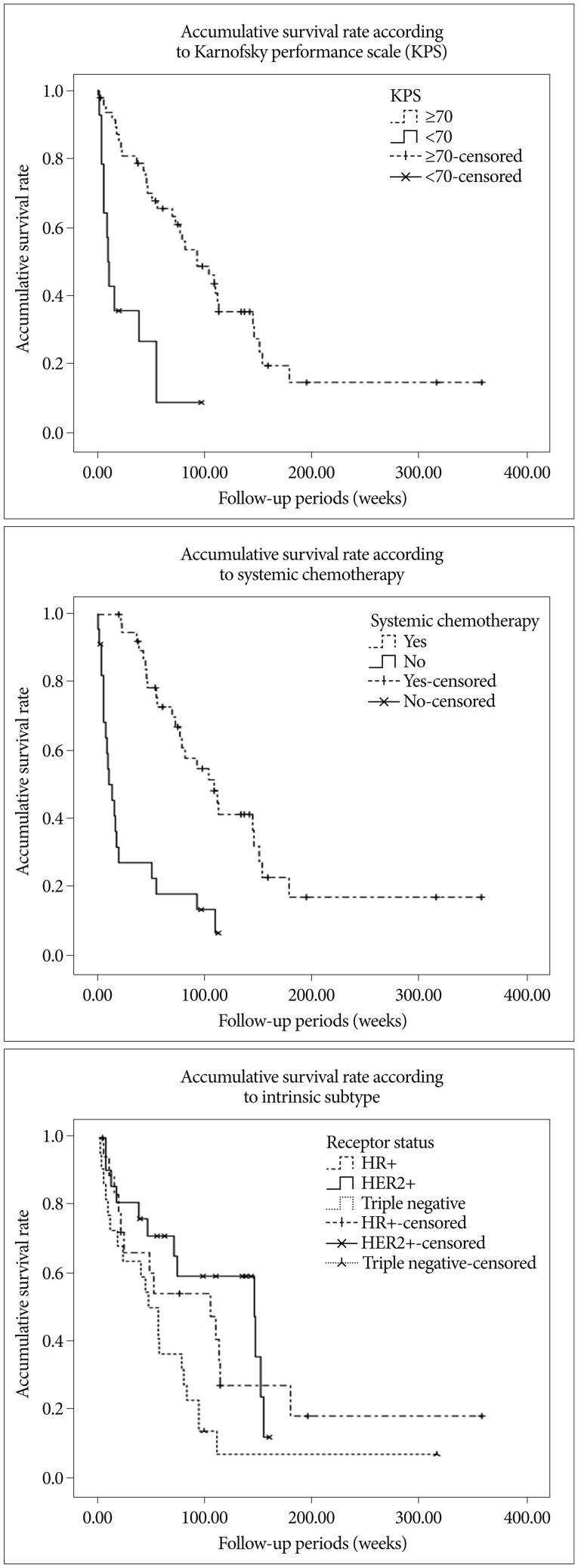
Fig.В 2
Actuarial distant control rate by breast cancer intrinsic subtype (p=0.327). 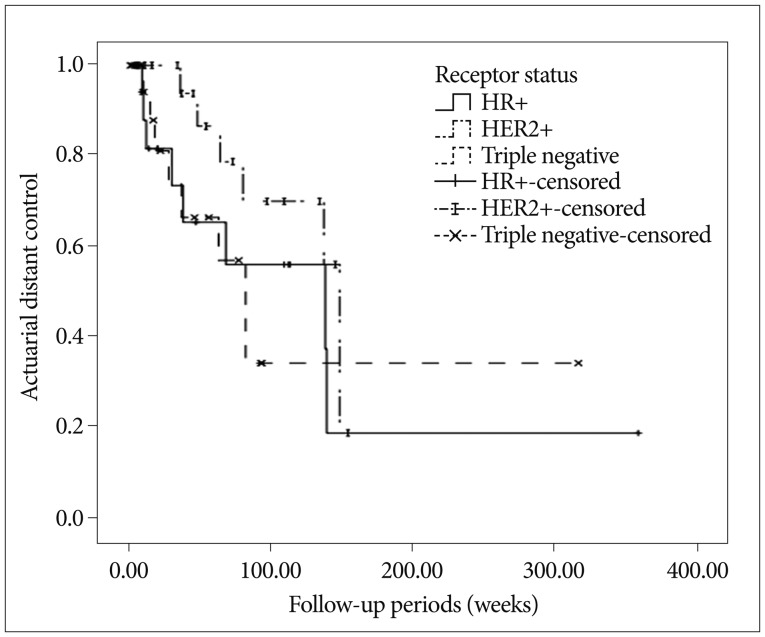
TableВ 1
TableВ 2
Summary of patient details by breast cancer subtype 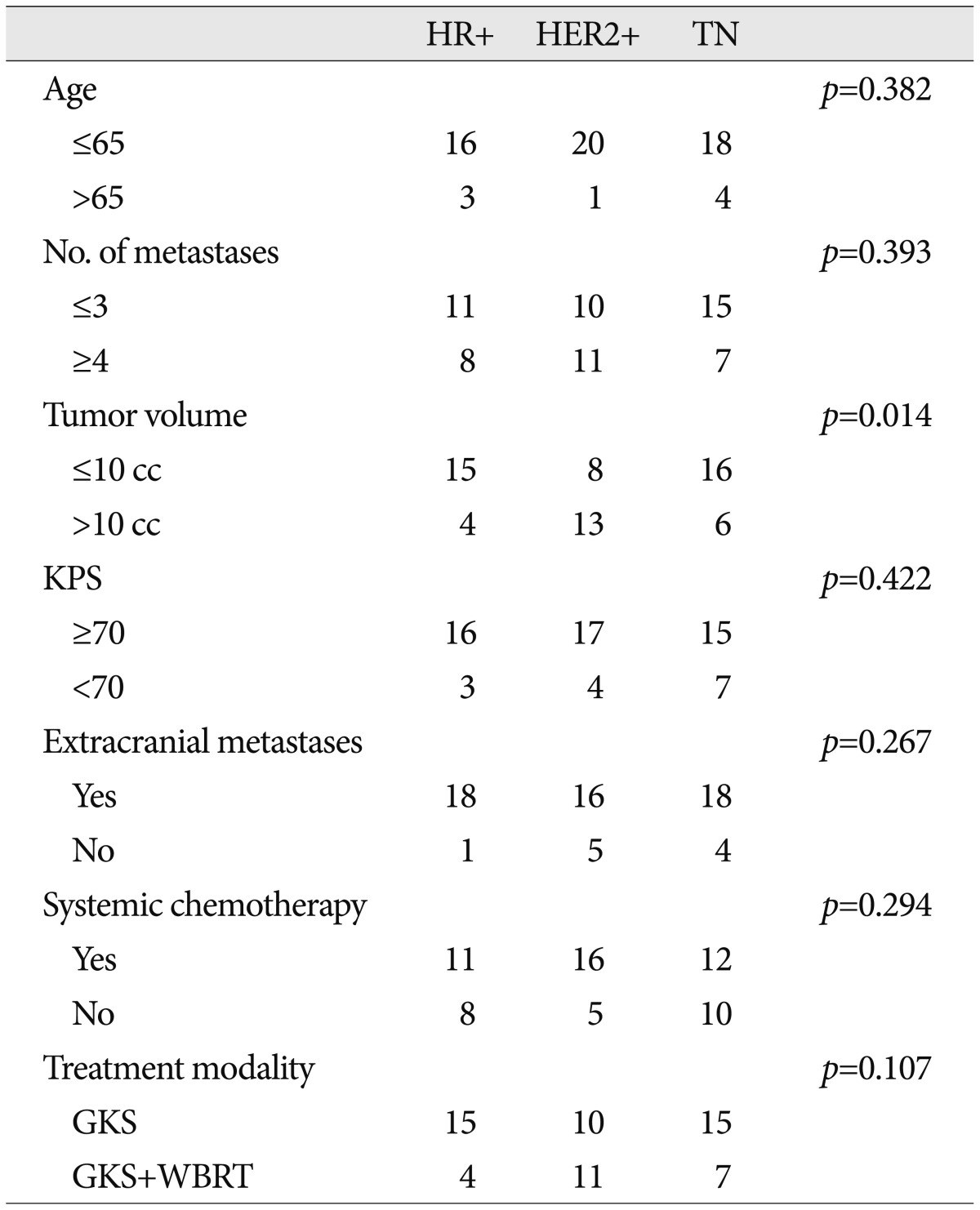
TableВ 3
Uni- and multivariate analysis results for overall survival 
TableВ 4
Uni- and multi-variate analysis results for local control 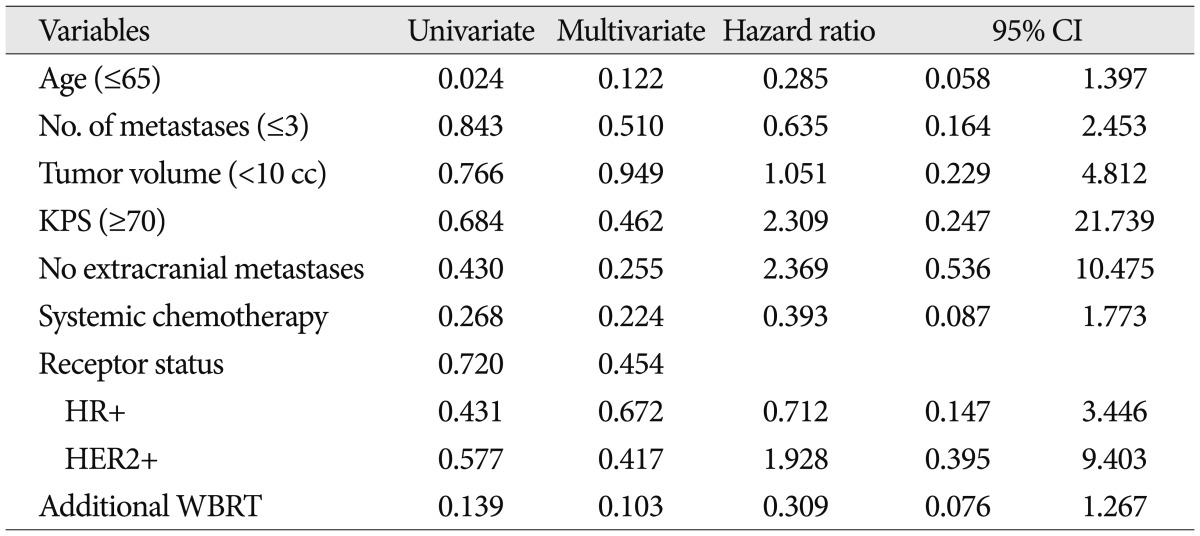
TableВ 5
Uni- and multi-variate analysis results for distant control 
|
|






















