INTRODUCTION
Intracranial aneurysms are found in 3.7 to 7.4% of patients with pituitary adenomas. Co-incidental aneurysms are detected almost seven times more frequently in patients with pituitary adenomas (PA) than in patients with other brain tumors2,12,19). However, the vast majority of these aneurysms are located outside the sellar-region. Aneurysms of the cavernous or supraclinoid carotid that encroach into the PA as in the present case are very rare. The prevalence of sellar-region's aneurysm among others is 1-2%14). The coexistance of intrasellar (not intracranial) aneurysms and PA is extremely rare. Treatments of these lesions are varied. Some possible treatment options include simultaneous microsurgical managements (frontopterional or supraorbital approach), transsphenoidal microsurgery after clipping or coil embolization, and two stage procedures combined with radiotherapy or radiosurgery (residual mass)2,13,14,19).
We report a patient with an incidental superior hypophygeal artery aneurysm embedded within a non-functional PA which was treated by transsphenoidal surgery after endovascular coil embolization.
CASE REPORT
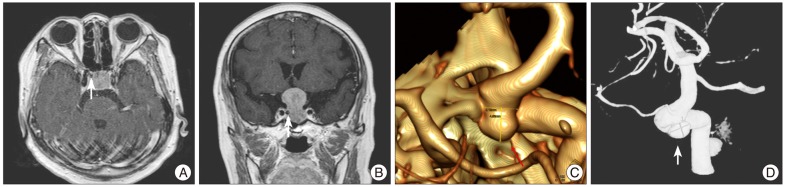
A 70-year-old woman presented with visual disturbance for one year. The results of the neuro-ophalmologiclal examination revealed bitemporal hemianopsia. Magnetic resonance (MR) imaging showed persistence of a homogeneously enhancing suprasellar lesion, compressing the visual pathways. Endocrine tests confirmed normal pituitary function. However, the axial or coronal MR images presented a flow void in the medial portion of the right internal carotid artery (ICA) (Fig. 1A, B). These MR images raised the suspicion of a coexisting intrasellar aneurysm before the planned transsphenoidal procedure. Consequently, 3-dementional computed tomography (CT) angiogram demonstrated the opthalmic segment of the right distal ICA (Fig. 1C). Four vessels angiogram confirmed a right superior hypophygeal artery aneurysm and the saccular aneurysmal sac that had a 4.13 mm neck, 6.27 mm width, and 3.99 mm height (Fig. 1D).
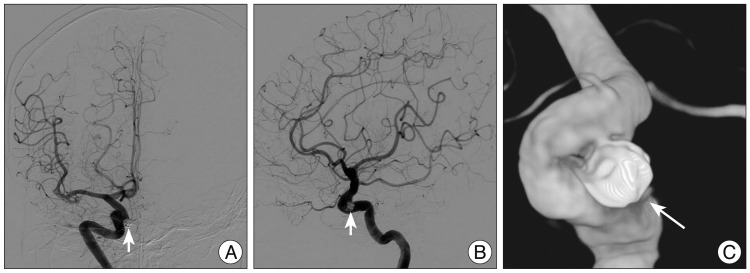
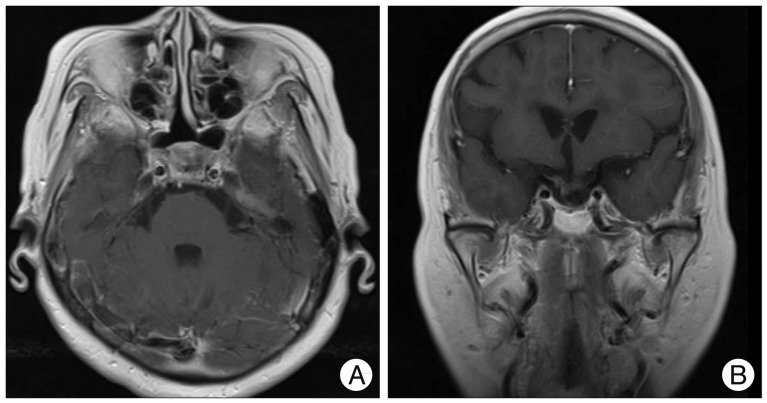
First, we treated the aneurysm by coil embolization with the goal of maintaining the patency of the ICA (Fig. 2). Two months later, she underwent surgery via the transsphenoidal microsurgical approach for the removal of the tumor. We carefully dissected the tumoral portion surrounding the coil-treated aneurysm and a total resection of the tumor was successfully achieved. The patient's postoperative course was uneventful. Immunohistological microscopic findings confirmed the diagnosis of a non-functional PA. Postoperative endocrine tests confirmed normal pituitary function. MR at 8 month follow-up after surgery demonstrated complete obliteration of the aneurysm and no sign of regrowth of the adenoma (Fig. 3).
DISCUSSION
The incidence of intracranial aneurysm among patients with PA is 0.5%, based on the results obtained from 5762 autopsies6). Since then, many studies have reported a 0.5-7.4% incidence of intracranial aneurysm among patients with PA, with a general consensus that this association is higher than the prevalence of intracranial aneurysm among the general patient population7,11,17). In addition, this association is generally thought to be higher in patients with PA compared with other brain tumors, although the manner in which a PA contributes to the formation of an intracranial aneurysm remains unclear5,17).
Oh et al.9) have reported that coexistence of intracranial aneurysm was detected in 18 of 800 patients with PA (2.3%). And then, the intracranial aneurysm in patients with PA were located in the internal carotid artery in 9 patients (50%), the middle cerebral artery in 6 patients (33.3%), the anterior cerebral artery in 2 patients (11.1%), and the vertebrobasilar artery in 1 patient (5.6%).
Hanak et al.4) have conducted a PubMed (National Library of Medicine) literature review to identify all studies reporting non-iatrogenic aneurysms with intrasellar extension, as confirmed by CT or MR imaging and angiography. Thirty-one studies reporting 40 cases of intrasellar aneurysms were identified. Eight aneurysms (20%) were diagnosed in conjunction with a PA.
Intrasellar aneurysms were reported in functional PA such as growth-hormone PA and prolactinoma9,12,14,18). But, the coexistence of a superior hypophygeal aneurysm and a non-functional pituitary adenoma was reported in one case so far19).
Direct infiltration by the tumor, and increased tension or blood flow in vessels supplying the tumor have been suggested as mechanisms for this coexistence of intracranial aneurysm and PA12). Approximately 50% of these patients have acromegaly, suggesting that high GH and IGF-1 levels or their biological effects may be implicated in the genesis of aneurysms. A high level of IGF-1 induces artery dilation, atherosclerotic and degenerative changes of the artery walls of the circle of Willis, tumor invasion and tumor-directed neovessels1,9). However, a clear mechanism of coexistence of two pathologies has not found.
These intrasellar aneurysms mimicking pituitary tumors are usually asymptomatic, although they can sometimes present with hypopituitarism (fewer than 40 cases in the literature). Aneurysm rupture or pituitary apoplexy is extremely rare16). In most cases, aneurysms are diagnosed incidentally during the preoperative workup of PA. Nevertheless, different clinical presentations may occur such as pituitary apoplexy or fatal epistaxis as a result of aneurysmal bleeding into the adenoma. Misdiagnosis of such coexistence can cause hazardous hemorrhagic complications7,14).
Teng et al.15) demonstrated that the finding of flow voids is 100% specific for aneurysms. However, the sensitivity of the presence of flow voids on noncontrast T1-weighted imaging, postcontrast T1-weighted imaging, and T2-weighted imaging was 88%, 22%, and 88%, respectively. Furthermore, Olsen et al.10) observed that only 12 of 15 giant aneurysms (80%) showed signs of intraluminal blood flow. These results indicate that misdiagnosis of such coexistence can be possible.
In our case, the axial or coronal MR images presented a flow void in the medial portion of the right ICA. Thus, 3-dimentional CT angiogram and 4 vessels angiogram were performed and confirmed a right superior hypophyseal artery aneurysm.
Incidental intracranial aneurysms located distant from the lesion are considered to have little relevance to surgical management of most patients. However, aneurysms of the major arteries adjacent to pituitary and suprasellar tumors are additional hazards to surgical treatment. Previous knowledge of the presence and anatomy of such aneurysms is important during separation of the tumor capsule from major vessels, or removal of an intracavernous extension of a tumor lying close to the carotid artery3,7).
Treatment of each lesion becomes more challenging when the aneurysm lies inside the sella turcica. Endovascular coil placement is an effective treatment option if it is performed before resection of the adenoma, particularly in locations such as the cavernous carotid artery or the paraclinoid segment.
Transsphenoidal microsurgical removal of the adenoma can still be performed safely in a subsequent surgical setting, but avoidance of direct contact with the aneurysm is suggested14,19).
The size and shape of the tumor would be important factors in the choice of treatment. If the tumor is smaller than a type A suprasellar (Hardy classification), endovascular coil embolization followed by transsphenoidal microsurgical removal of the PA may be feasible and beneficial18). If it is not possible to achieve a total resection of PA without manipulating the aneurysm via the transsphenoidal route, simultaneous microsurgical management may be a better treatment option2). In addition, adjuvant radiotherapy or radiosurgery will be considered if recurrent tumor is found during follow up8). In our case, we treated firstly the aneurysm by coil embolization and PA by the transsphenoidal microsurgical approach two months later.
CONCLUSION
The presence of an intrasellar aneurysm embedded within a pituitary tumor is extremely rare.
We report a patient with an incidental superior hypophygeal artery aneurysm embedded within a non-functional PA. Therefore, careful evaluation of pre-operative imaging is necessary, especially in those with atypical symptoms such as our patient. Prior treatment of the aneurysm is advisable to avoid catastrophic bleeds during transsphenoidal microsurgical removal of the adenoma.