Kim, Lim, Oh, Kim, Kim, and Shin: Thromboembolic Events Associated with Electrolytic Detachment of Guglielmi Detachable Coils and Target Coils : Comparison with Use of Diffusion-Weighted MR Imaging
Abstract
Objective
The purpose of this study was to retrospectively evaluate and compare the incidence of diffusion-weighted image (DWI) lesions between the Guglielmi detachable coil (GDC) and the Target coil for treating unruptured intracranial aneurysm.
Methods
From 2010 to 2011, consecutive 222 patients with an intracranial aneurysm underwent coil embolization. Inclusion criterias were : 1) unruptured intracranial aneurysm, 2) one or more GDC or Target coils used with or without other coils, 3) DWI examination within 24 hours after coiling, and 4) coiling performed without a balloon or stent.
Results
Ninety patients (92 cases) met the inclusion criteria. DWI lesions were detected in 55 (61.1%) of 90 patients. In the GDC group (n=44), DWI lesions were detected in 31 (70.5%). The average number of DWI lesions was 5.0Вұ8.7 (meanВұSD; range, 1-40) in aneurysm-related territory. In the Target coil group (n=48), DWI lesions were detected in 24 (50.0%). The number of DWI lesion was 2.1Вұ5.4 (range, 1-32) in aneurysm-related territory. There was no significant correlation between a number of coils and DWI lesions. No significant differences were also observed in the number of DWI lesions in each group.
Conclusion
The GDC and Target coils, which have an electrolytic detachable system, showed no differences in the incidence of DWI lesion.
Key Words: Air bubble В· Coil embolization В· Diffusion-weighted imaging В· Detachable coil В· Thromboembolism.
INTRODUCTION
The Guglielmi detachable coil (GDC) is the first detachable coil approved by the Food and Drug Administration of the United States for treating intracranial aneurysms 4,7). Although the GDC has been demonstrated as safe and effective for endovascular treatment of intracranial aneurysms, there have been several reports concerning thromboembolic events related to GDC use due to air bubbles and electrothrombosis when it is electrolytically detached. Han et al. 4) reported that the electrolytic detachment mechanism of the GDC generated gas bubbles and thrombi from the coil detachment zone and this phenomenon may be a potential cause for thromboembolic complications during treatment of cerebral aneurysms. The Target detachable coil (Target; Stryker, Fremont, CA, USA) has been introduced recently in our country. Target coils have the same electrolytic detachment mechanism as a GDC and we had expected the new advanced coils would generate less bubbles than the conventional GDCs during the detachment. Unfortunately, according to our in vitro experiments, the Target coils generate larger-sized and more considerable number of air bubbles than the GDCs ( Fig. 1). Recently, Lee et al. 8) reported that the air bubbles were larger-sized and generated most frequently in Target coil system than other coils through their in vitro experiments. Introduction and detachment of the coils may take a share of the procedure after manipulation of microcatheter with microwire. Our authors were wondering the air bubbles from the coils matter seriously to patients underwent coil embolization for intracranial aneurysm. However, there was no study for clinical correlation of the air bubbles as thromboembolic source although some authors gave a warning for formation of air bubbles from the electrolytic detachable coils, especially the GDCs or Target coils.
The purpose of this study was to retrospectively evaluate and compare the incidence of diffusion-weighted imaging positive lesion (DWIL)-as practical obtainable finding-between two electrolytic detachable coils (GDC versus Target coil) for treating unruptured intracranial aneurysm (UIA).
MATERIALS AND METHODS
Patients
From March 2011 to December 2011, we performed endovascular embolization for intracranial aneurysms using one or more Target coils for 99 cases in 94 patients. There were 24 men and 70 women (age, 59.3Вұ10.0 years; range, 40-82 years). Sixty-eight (68.2%) unruptured aneurysms occurred in 99 cases. Eighty-one (81.8%) cases were located in the anterior circulation, including the internal carotid artery, 31; posterior communicating artery, 16; middle cerebral artery, 14; anterior communicating artery, 13; anterior cerebral artery, five; and 18 cases in the posterior circulation, including seven vertebral artery, three posterior inferior cerebellar artery, one anterior inferior cerebellar artery, six basilar artery, and one posterior cerebral artery. The average size of the aneurysms was 6.56Вұ3.30 mm (range, 2.6-24.6 mm). We performed stent-assisted coil embolization in 18 (18.2%) and balloon-assisted in six (6.1%) of 99 cases.
The historical control group included 132 consecutive patients, harboring 135 aneurysms, who underwent coil embolization using one or more GDCs during the period from January 2010 to March 2011 before the Target coil was launched.
Inclusion criteria were: 1) UIA, 2) one or more GDC or Target coils in use with or without other coils, 3) diffusion-weighted image (DWI) examination within 24 hours after coiling, and 4) coiling performed without a balloon or stent. Among 222 patients, ninety patients (92 cases) met the inclusion criteria. The patients were subdivided into a GDC-treated group (n=44) and a Target-coil treated group (n=48) ( Fig. 2). The aneurismal locations are summarized in Table 1.
Endovascular treatment
All procedures were performed via a transfemoral route with general anesthesia. Flushed saline and contrast medium were heparinized (1000 IU/100 mL), and both a guiding catheter and a microcatheter were placed for a continuous heparinized drip. All patients were administered systemic heparinization (loading dose of 3000 IU followed by 1000 IU/hour) during the procedure. The systemic heparinization was discontinued at the end of the procedure and not reversed. All treated aneurysms were evaluated angiographically after embolization by our neurovascular team. The results of the embolization were classified into complete (no filling of aneurismal rests without a neck remnant), incomplete occlusion (saccular contrast material filling) and neck remnant. All procedure records were reviewed for the number of GDCs or Target coils and the total other detached coils.
DWI evaluation
Magnetic resonance imaging was performed within 24 hours after the coiling procedure in all cases to check for any silent thromboembolic events, regardless of neurological changes. Imaging was performed with 1.5-T system (Signa HDxt; GE Medical Systems, Milwaukee, WI, USA) and a 3.0-T system (Magnetom Verio; Siemens, Erlangen, Germany). All MR images were reviewed by two neurointerventionalists (B.-s.K, M.J.K). If DWI abnormalities were detected, their number and location were recorded. Aneurysm-related lesions was defined as finding location in the vascular territory downstream from the treated aneurysm, in another cases they were checked as aneurysm-unrelated lesions (i.e., anterior circulation versus posterior circulation, ipsilateral hemisphere territory versus contralateral hemisphere territory except lesion of anterior communicating artery including both territories).
Statistical analysis
The demographic characteristics of the aneurysms, coiling results, and lesions on DWI were compared between the GDC and Target coil groups using a contingency table or mean comparison. Correlations between multiple DWILs (>10 dots) and distinguished risk factors in the groups were analyzed by logistic regression. We conducted partial correlations analysis to show the statistical differences of continuous variable between DWILs and each coil ratio, and adjusted by confound factors. All statistics were performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA), and a p-value <0.05 was considered significant.
RESULTS
DWI was performed in all 90 patients (92 cases; two patients underwent coil embolization for aneurysms located on different sites) after the coil embolization. DWILs were detected in 55 (61.1%) of 90 patients. However, all patients with DWIL were asymptomatic except for one patient who underwent coil embolization using three GDCs for an unruptured posterior inferior cerebellar artery (PICA) aneurysm and suffered headache and dizziness after the procedure. Several DWILs were detected in ipsilateral PICA territory ( Fig. 3). The symptoms improved completely at discharge. DWILs were detected in 31 (70.5%) of 44 cases in the GDC group. The average number of DWILs was 5.0Вұ8.7 (range, 1-40) in aneurysm-related territory and 0.8Вұ1.2 (range, 1-4) in aneurysm-unrelated territory. In the Target coil group, DWIL were detected in 24 (50.0%) of 48 cases after the procedure. The number of lesions was 2.1Вұ5.4 (range, 1-32) in aneurysm-related territory and 1.4Вұ3.1 (range, 1-14) in aneurysm-unrelated territory ( Table 2). The Target coil group tended to reveal fewer aneurysm-related DWILs, however, it was not significant. No association between multiple DWILs with high signal dots over 10 and any of the risk factors (involving group, aneurismal size and occlusion results) was found ( Table 3). A ratio of the GDCs in total coils was 45.8% (range, 10.0-100%) and the Target coils were 67.8% (range, 15.4-100%) in each the GDC/Target coil group. There was no significant correlation between the number of DWILs and a ratio of the coils in GDCs or Target group ( Fig. 4).
DISCUSSION
Since the endovascular treatment using GDCs was first introduced in 1991, several coil materials have been developed and used for treating intracranial aneurysms. The safety and efficacy of the endovascular technique using coil materials have been reported by several authors 11,15,19). In particular, the coils improved rapidly, which made it possible to improve anatomic and clinical outcomes of endovascular coiling 9,11,12,19). Although the treatment has world-wide acceptance for intracranial aneurysms, thromboembolic complications still remain as procedure-related main events 10,14,17). Several studies have shown that thromboembolic events including stroke and transient ischemic attacks occur at a rate of 2.4-28% 3,10,14). Those series defined complications as new focal neurological deficits, mentality changes, or abnormal findings detected on a post-procedural imaging study. Pelz et al. 14) reported that thromboembolic events associated with conventional coil embolization using GDCs occurred in 28% of cases, with permanent deficits affecting approximately 5% in their study group. Rordorf et al. 16) detected embolic infarctions that were related to the coil placement procedure in eight (61%) of 14 patients, and they were all asymptomatic. Soeda et al. 18) reported that DWI showed high signal lesions in 40 (61%) of 66 patients, with 16 patients (40%) eventually developing neurological symptom. In our series, a symptomatic thromboembolism occurred in only one (1.1%) of 90 patients; however, new high signal lesions on DWI were detected at the aneurysm-related territory in 61.1% (55/90) of patients without neurological symptoms, suggesting silent thromboembolic events. These results reveal unpredictable rates of thromboembolic events related to coil embolization using GDCs. Thromboembolic events may be caused by thrombus formation from the delivery system, endosaccular thrombosis, protruding coils into the parent vessel, or other supporting devices for endosaccular stabilization of the coils 5). Some studies have documented that balloon- or stent-assisted coil embolization increases thromboembolic risk, but this remains controversial 1,5). Several authors have reported that thromboembolic events are associated with GDC embolization and the use of DWI 6,17,18). Among the causative factors, Rordorf et al. 16) suggested that air bubbles in the fusion solutions may act as emboli, and Padolecchia et al. 13) and Han et al. 4) investigated the role of electrothrombosis in embolizations performed with GDCs. They showed that thrombus formation occurred at the detachment zone in their experimental environments. These studies suggest that thrombi might form during the procedure, as well as immediately after detachment of the coils. The clots that form during the detachment process around the detachment zone or adjacent to the coils may be dislodged during withdrawal of the delivery wire, repositioning of the microcatheter, or repetitive endosaccular introduction of the coil 1). The GDC, which is the most commonly used electrolytic detached coil system, has frequent thromboembolic complications due to its detachment system 4,13,16). The Target coil, which is a new version of the GDC, has been in use since early 2011. Several changes and improvements have been implemented over the conventional GDCs. However, according to our in vitro experiment and microscopic examination of Target coils, air bubbles were still observed when it is electrolytically detached ( Fig. 1). In addition, the air bubbles from detach zone of the coils were considerable amount more than the conventional GDCs on the contrary our expectation. In our results, there was no significant correlation between the ratio of GDC and/or Target coils and detected high signal dots on DWI ( p=0.704). The number of electrolytic detachable coils was not significant for the number of DWIL between the two groups. Considering these results adjusted by confounding factors (aneurismal size and occlusion results), we suggest that the electrolytic detached coil system may be not a significant factor for thromboembolic events. Nevertheless, as electrothrombosis during embolization occurs with GDCs 4,13), our results also revealed many cases with DWIL. However, the relationship between the electrolytic detachable system and DWIL was unclear, and all cases were silent events except one case with mild neurological deterioration. Our author reviewed the results in only single GDC (n=5), Target (n=17) or other kind (n=6) of coils-using 28 cases in spite of a few cases. There were also no significant correlation between the number of coils and the DWILs (4.2Вұ3.7; 1.5Вұ2.1; 0.7Вұ0.8, p=0.104).
No significant difference was observed between the Target coil group and the GDC group for aneurysm-related DWIL. Manufacturers officially announced that the Target detachable coil is an advanced model of the GDC coil detachment system. However, Target detachable coils still have the electrolytic detachable system and may be not superior to previous generation GDCs for electrothrombosis during conventional coil embolization.
As high signal dots in DWI were also detected in the aneurysm-unrelated territory, DWILs were not always located in the vascular territory of a parent artery of the aneurysm. Lesions can develop from collateral flow through the anterior or posterior communicating artery or from other sites before the coil embolization procedure 2,17).
Limitations of our study
The choice of coil material was likely to be influenced by practitioner's preference at that time during procedure. In addition, we acknowledge that our study is a retrospective analysis with a selection bias because of inclusion of the results in cases used few GDCs or Target coils-even if at least the coil was used-to our data. It may be unreasonable to draw exactly the effect of the electrolytic detachable system on the rate of ischemic events on DWI because some other factors (e.g., catheter and coil manipulation, procedural time and experience of practitioner) could affect the environment. Therefore, in this study, to exclude the other factors, relatively narrow inclusion criteria (unruptured aneurysm, no supporting devices and simple technique) were applied. Moreover, total Target detachable coils were used more than one third and the semi-results only involving the GDC or Target coils more than fifty percentage were parallel to the overall result. Therefore, our results suggest that it may support in a degree the relation between electrolytic detachable coils and DWILs. However, for the more significant data coincided with our purpose, prospective study should be performed using unitary detachable coils under unitary environment except other factors.
CONCLUSION
Our study has shown a relatively high prevalence of DWIL that occurred after coil embolization for UIA using GDCs/Target electrolytic detachable coils. No difference in the incidence of DWIL was observed between the GDC and the newly developed Target coil in a similar environment. Furthermore, the DWILs were almost silent without permanent clinical sequelae. Our results suggest that electrolytic detachment of the coil may not be a significant contributor to the incidence of DWIL during endovascular coil embolization.
References
1. Albayram S, Selcuk H, Kara B, Bozdag E, Uzma O, Kocer N, et al : Thromboembolic events associated with balloon-assisted coil embolization: evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2004, 25 : 1768-1777,   2. Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L : Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999, 354 : 1594-1597,   3. Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F : Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 1998, 43 : 1281-1295; discussion 1296-1297,   4. Han MH, Kwon OK, Yoon CJ, Kwon BJ, Cha SH, Chang KH : Gas generation and clot formation during electrolytic detachment of Guglielmi detachable coils: in vitro observations and animal experiment. AJNR Am J Neuroradiol 2003, 24 : 539-544,   5. Hwang G, Park H, Bang JS, Jin SC, Kim BC, Oh CW, et al : Comparison of 2-year angiographic outcomes of stent- and nonstent-assisted coil embolization in unruptured aneurysms with an unfavorable configuration for coiling. AJNR Am J Neuroradiol 2011, 32 : 1707-1710,    6. KlГ¶tzsch C, Nahser HC, Henkes H, KГјhne D, Berlit P : Detection of microemboli distal to cerebral aneurysms before and after therapeutic embolization. AJNR Am J Neuroradiol 1998, 19 : 1315-1318,   7. Koebbe CJ, Veznedaroglu E, Jabbour P, Rosenwasser RH : Endovascular management of intracranial aneurysms: current experience and future advances. Neurosurgery 2006, 59( 5 Suppl 3):S93-S102; discussion S3-S13,   8. Lee DH, Hwang SM, Lim OK, Kim JK : In vitro observation of air bubbles during delivery of various detachable aneurysm embolization coils. Korean J Radiol 2012, 13 : 412-416,    9. Moret J, Cognard C, Weill A, Castaings L, Rey A : The "Remodelling Technique" in the Treatment of Wide Neck Intracranial Aneurysms. Angiographic Results and Clinical Follow-up in 56 Cases. Interv Neuroradiol 1997, 3 : 21-35,   10. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al : Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg 2003, 98 : 959-966,   11. Murayama Y, ViГұuela F, Duckwiler GR, Gobin YP, Guglielmi G : Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg 1999, 90 : 207-214,   12. Nelson PK, Levy DI : Balloon-assisted coil embolization of wide-necked aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. AJNR Am J Neuroradiol 2001, 22 : 19-26,   13. Padolecchia R, Guglielmi G, Puglioli M, Castagna M, Nardini V, Collavoli PL, et al : Role of electrothrombosis in aneurysm treatment with Guglielmi detachable coils: an in vitro scanning electron microscopic study. AJNR Am J Neuroradiol 2001, 22 : 1757-1760,   14. Pelz DM, Lownie SP, Fox AJ : Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998, 19 : 1541-1547,   15. Qureshi AI, Suri MF, Khan J, Kim SH, Fessler RD, Ringer AJ, et al : Endovascular treatment of intracranial aneurysms by using Guglielmi detachable coils in awake patients: safety and feasibility. J Neurosurg 2001, 94 : 880-885,   16. Rordorf G, Bellon RJ, Budzik RE Jr, Farkas J, Reinking GF, Pergolizzi RS, et al : Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 2001, 22 : 5-10,   17. Sakai H, Sakai N, Nakahara I, Shimozuru T, Higashi T, Takahashi JC, et al : Embolic Complications of Endovascular Surgery for Cerebrovascular Diseases. Evaluation with Diffusion-Weighted MR Imaging. Interv Neuroradiol 2000, 6 Suppl 1 : 223-226,   18. Soeda A, Sakai N, Sakai H, Iihara K, Yamada N, Imakita S, et al : Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003, 24 : 127-132,   19. ViГұuela F, Duckwiler G, Mawad M : Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997, 86 : 475-482,  
Fig.В 1
Microscopic photographs of electrolytic detachment process of GDC-10 SynerG (A) and Target detachable coil (B) in heparinized saline. Multiple and variable sized bubbles are generated around the detachment zone. GDC: Guglielmi detachable coil. 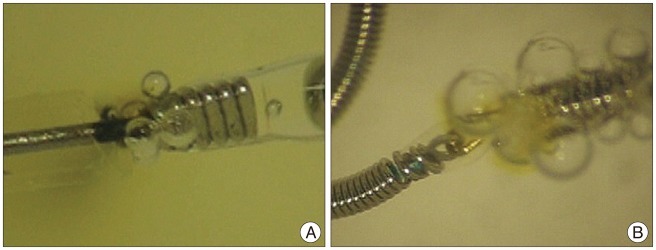
Fig.В 2
Flow diagram for patient selection. GDC: Guglielmi detachable coil, DWI: diffusion-weighted image. 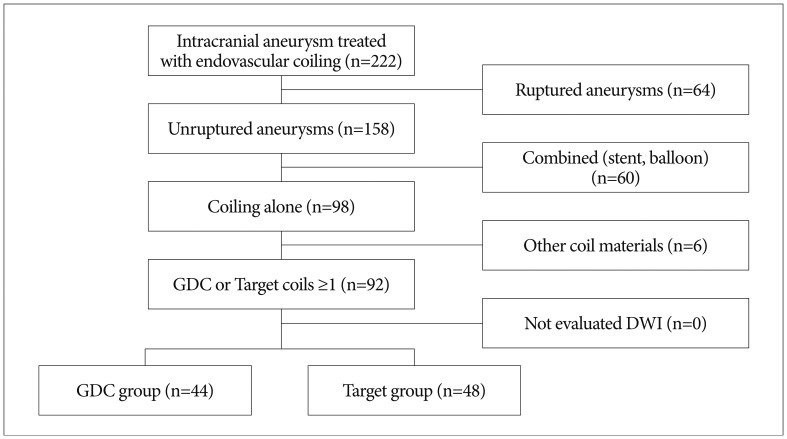
Fig.В 3
A: A 53-year-old female patient with an unruptured left PICA aneurysm sized about 4 mm on a three-dimensional reconstruction image. B: The coil embolization using three GDCs without supporting materials has been performed completely for the aneurysm. C and D: Several high signal dots are detected in ipsilateral PICA territory on DWI. PICA: posterior inferior cerebellar artery, GDC: Guglielmi detachable coil, DWI: diffusion-weighted image. 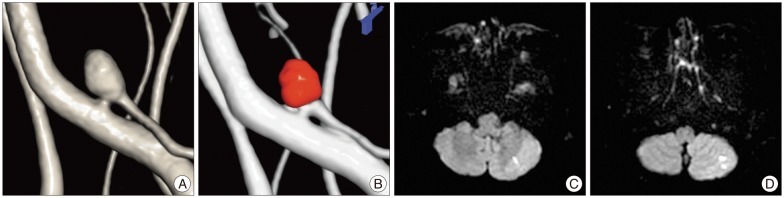
Fig.В 4
The number of the aneurysm-related DWILs and coil embolization results for the aneurysms based on number of the GDC (A) or Target coil (B) of total detachable coils. There is no significance between A and B (p=0.704). Partial correlation coefficient (r) adjusted by aneurismal size and occlusion results. DWIL: diffusion-weighted imaging positive lesion. DWIL: diffusion-weighted imaging positive lesion, GDC: Guglielmi detachable coil. 
TableВ 1
Distribution of aneurysms treated with coil embolization 
TableВ 2
Univariate comparison between the Guglielmi detachable coil (GDC) group and the Target coil group 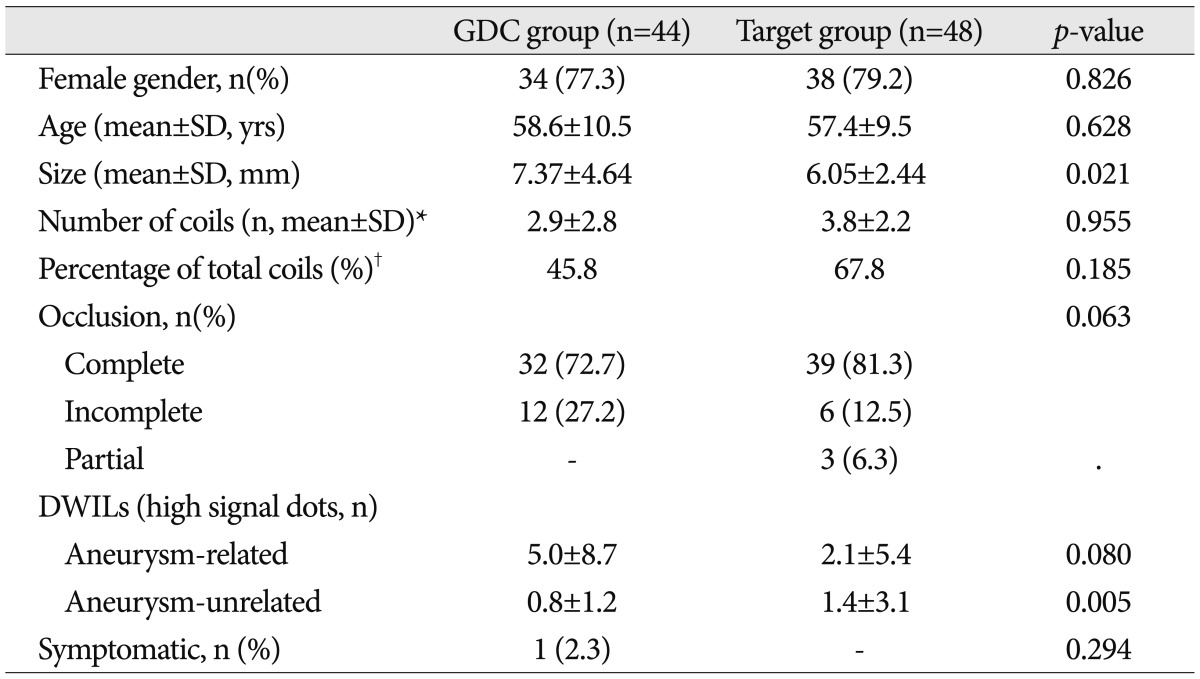
TableВ 3
Predictable risk factors for multiple embolic (high signal dots >10) infarction after coil embolization by logistic regression analysis 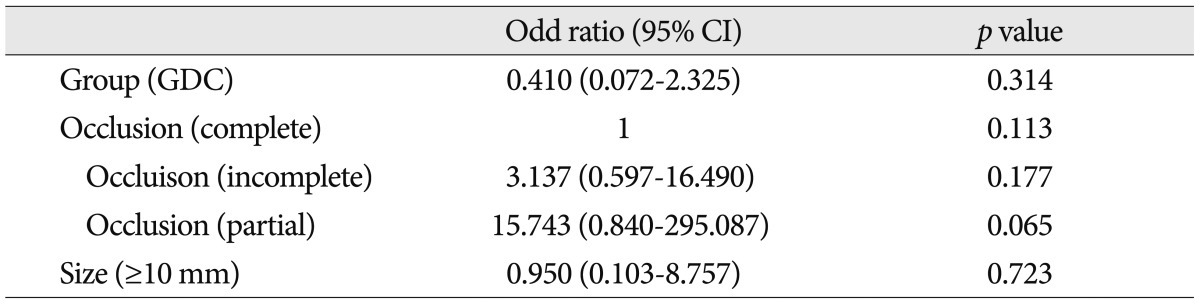
|
|






















