Kwon, Ko, Kwon, Kim, and Lee: Evaluation of Stent Apposition in the LVIS Blue Stent-Assisted Coiling of Distal Internal Carotid Artery Aneurysms : Correlation with Clinical and Angiographic Outcomes
Abstract
Objective
To evaluate the stent apposition of a low-profile visualized intraluminal support (LVIS) device in distal internal carotid artery (ICA) aneurysms, examine its correlation with clinical and angiographic outcomes, and determine the predictive factors of ischemic adverse events (IAEs) related to stent-assisted coiling.
Methods
We retrospectively analyzed a prospectively maintained database of 183 patients between January 2017 and February 2020. The carotid siphon from the cavernous ICA to the ICA terminus was divided into posterior, anterior, and superior bends. The anterior bends were categorized into angled (V) and non-angled (C, U, and S) types depending on the morphology and measured angles. Complete stent apposition (CSA) and incomplete stent apposition (ISA) were evaluated using unsubtracted angiography and flat-panel detector computed tomography. Dual antiplatelet therapy with aspirin 200 mg and clopidogrel 75 mg was administered. Clopidogrel resistance was defined as fewer responders (Ōēź10%, <40%) and non-responders (<10%) based on the percent inhibition (%INH) of the VerifyNow system. These were counteracted by a dose escalation to 150 mg for fewer responders or substitution with cilostazol 200 mg for non-responders. IAEs included intraoperative in-stent thrombosis, transient ischemic attack, cerebral infarction, and delayed in-stent stenosis. A multivariate logistic regression analysis was used to determine the predictive factors for ISA and IAEs.
Results
There were 33 ISAs (18.0%) and 27 IAEs (14.8%). The anterior bend angle was narrower in ISA (-4.16┬░┬▒25.18┬░) than in CSA (23.52┬░┬▒23.13┬░) (p<0.001). The V- and S-types were independently correlated with the ISA (p<0.001). However, treatment outcomes, including IAEs (15.3% vs. 12.1%), aneurysmal complete occlusion (91.3% vs. 88.6%), and recanalization (none of them), did not differ between CSA and ISA (p>0.05). The %INH of 27 IAEs (13.78%┬▒14.78%) was significantly lower than that of 156 non-IAEs (26.82%┬▒20.23%) (p<0.001). Non-responders to clopidogrel were the only significant predictive factor for IAEs (p=0.001).
Conclusion
The angled and tortuous anatomical peculiarity of the carotid siphon caused ISA of the LVIS device; however, it did not affect clinical and angiographic outcomes, while the non-responders to clopidogrel affected the IAEs related to stent-assisted coiling.
Key Words: Carotid siphon ┬Ę Clopidogrel resistance ┬Ę Low-profile visualized intraluminal support ┬Ę Stent apposition ┬Ę Stent-assisted coiling.
INTRODUCTION
The low-profile visualized intraluminal support (LVIS) (MicroVention, Tustin, CA, USA) device is a 3rd-generation self-expanding stent developed for stent-assisted coiling (SAC) of wide-neck aneurysms, with a new modification named LVIS Blue launched in 2017 [ 20]. It has a high metal coverage (19-28%) approximating flow diverters (30-35%), resulting in an improved reduction of inflow rate into the aneurysm [ 5, 16, 27], making for excellent aneurysmal occlusion performance [ 3, 15, 23, 33]. Four flared radiopaque markers on both ends and two visualized helical strands within the body allow for a real-time assessment of stent deployment. Furthermore, its closed-cell design allows retrieval and repositioning for up to 3 mm of a stent within the catheter. Most importantly, the LVIS Blue is designed to have a refined radial force and conformability, which improves stent apposition within the vessel wall [ 4, 22]. However, since the device is fundamentally a wire-braided stent where the surgeonŌĆÖs skill can affect stent deployment, concerns about poor wall apposition in the tortuous vessel, especially in the carotid siphon, which has been experienced in the previous LVIS version, may not be easily resolved [ 3, 7]. Although recent reports examined the stent apposition of LVIS Blue in vitro [ 4, 14], its frequency, cause, and adverse effects remain unknown in practical clinical settings. Therefore, we evaluated the stent apposition of the LVIS device in distal internal carotid artery (ICA) aneurysms, examined its correlation with clinical and angiographic outcomes, and determined the predictive factors of ischemic adverse events (IAEs) related to SAC.
MATERIALS AND METHODS
Study population
After the approval of the Institutional Review Board, a total of 311 patients with unruptured saccular distal ICA aneurysms who visited our institution between January 2017 and February 2020 were subjected to endovascular treatment. Among them, 195 patients, except for those with narrow neck aneurysms suitable for coiling alone (n=91), wide-neck aneurysms treated with the two-catheter technique or balloon-assisted coiling (n=19), and giant aneurysms with a flow-diverting stent (n=6), were eligible for SAC using the LVIS device without the application of any other stent. Patients without a clinical follow-up of >1 year (n=5) or who could not undergo the required radiological examination (n=7) were excluded. Finally, we enrolled 183 consecutive patients treated with LVIS Blue for 38 months.
We performed a retrospective analysis of a single center using a prospectively maintained database that contained factors potentially related to stent apposition. Medical records such as sex, age, smoking, alcohol, hypertension, diabetes mellitus, dyslipidemia, previous hemorrhage or infarction, and prior antiplatelet medication were collected accordingly. All patients underwent digital subtraction angiography (DSA), and their aneurysm configurations, such as maximal diameter, dometo-neck ratio, and aspect ratio, were evaluated. The location of the aneurysm was classified as paraclinoid aneurysm, including the ophthalmic artery, superior hypophyseal artery, paraclinoid dorsal wall, and clinoid segment aneurysm, and supraclinoid aneurysm, including the posterior communicating artery and anterior choroidal artery aneurysm.
Classification of carotid siphon
Three major curves in the carotid siphon from the cavernous ICA to the ICA terminus were named the posterior, anterior, and superior bends, defined by intersecting lines through the proximal vertical segment of the cavernous ICA, horizontal segment of the cavernous ICA, ophthalmic ICA, and communicating ICA, respectively. The angle of each bend was measured at the intersection between two lines traced through the midpoints of the diameters of each straight segment, and the angle measurements were performed on the images reflecting the plane formed by these two straight lines.
If the lines through the horizontal segment of the cavernous ICA and the ophthalmic ICA crossed posteriorly to the carotid siphon due to its tortuosity, the numerical value of the anterior bend was measured as a negative value. In particular, the anterior bend of the carotid siphon was categorized into angled (V) and non-angled types (C, U, and S), according to the classification proposed by Zhang et al. [ 31]. The non-angled type was divided into C (Ōēź20┬░), U (Ōēź-20┬░, <20┬░), and S (<-20┬░) according to the values of the measured angles ( Fig. 1). We also measured the maximum and minimum ICA diameters in the range predicted as the stent deployment site.
Antiplatelet regimen
Dual antiplatelet therapy (DAPT) was administered as a first-line treatment for all patients scheduled for SAC. We prescribed aspirin 200 mg and clopidogrel 75 mg daily for 7 days prior to the procedure. Platelet inhibition tests were performed using ASA and P2Y12 assays of the VerifyNow System (Accumetrics, San Diego, CA, USA) one day prior to the procedure. Aspirin resistance was defined as non-responders (Ōēź550 aspirin reaction unit [ARU]) based on the ARU. Clopidogrel resistance was defined as fewer responders (Ōēź10%, <40%) and non-responders (<10%) based on the percent inhibition (%INH), calculated as (A-B)/A├Ś100, which indicates the difference before (A) and after drug therapy (B) using the P2Y12 reaction unit (PRU).
An insufficient response to clopidogrel was counteracted by dose escalation to 150 mg for fewer responders or substitution with cilostazol 200 mg for non-responders during 2 weeks of post-procedure; subsequently, the adjusted dose of clopidogrel 75 mg or cilostazol 100 mg was maintained accordingly. Aspirin was reduced to 100 mg approximately 3 months after SAC, and DAPT was switched to life-long aspirin single antiplatelet therapy (SAPT) 1 year later. We maintained the planned regimen regardless of the status of stent apposition.
Deployment of LVIS stent
The diameter specifications of the selected LVIS stent were 4.0 mm (ICA <4 mm), 4.5 mm (ICA Ōēź4.0 mm and <5.5 mm), and 5.5 mm (ICA Ōēź5.5 mm) based on the measured maximal ICA diameter. Most procedures were conducted using biaxial access and the semi-jailing technique. The stent was submerged in a bowel of heparinized saline before being loaded into the microcatheter. Thereafter, it was partially deployed up to 50% and gently massaged to assist with full expansion. The stent was delivered to the desired site for deployment under roadmap guidance and was generally deployed from the posterior communicating artery in paraclinoid aneurysms and the ICA terminus in supraclinoid aneurysms. During stent deployment, when the two radiopaque strands insufficiently contacted the vessel wall, the push-pull technique was utilized to retrieve the malpositioned stent and redeploy the stent, while tension was provided by the push-back of the entire stent system.
If the stent exhibited an incomplete opening at the proximal flared end after being released from the microcatheter, a microwire with a J-shaped tip was gently massaged using back-and-forth motion to fully expand the stent. However, when the stent body lumen was folded due to a stent kink, stent adjustments, such as microwire massage and balloon angioplasty, were not performed due to the concern surrounding stent displacement or thromboembolism. Balloon angioplasty using the Scepter CTM double-lumen balloon catheter (MicroVention) was only applied in cases of an intraoperative rupture of the aneurysm to stop the bleeding ( Fig. 2).
Evaluation of stent apposition
Stent apposition to the vessel wall was evaluated via unsubtracted angiography in the anteroposterior, lateral, and working projections as well as flat-panel detector computed tomography (FDCT) with 20% diluted contrast medium using the XperCT protocol (Philips Health Care, Best, The Netherlands). The latter was visualized as high spatial resolution images through a multiplanar reconstruction in the maximal intensity projection mode with a 10 mm slice thickness. Therefore, complete stent apposition (CSA) was defined as the full expansion of the entire stent system. Incomplete stent apposition (ISA) was defined as the separation of one or more stent struts from the stent-containing arterial wall. This was classified as trunk malposition with a gap between the stent body and vessel and edge malposition with insufficient wall anchoring of the flared ends.
FDCT without a contrast medium was also performed to identify the degree of stent kinking and coil loop protrusion across the stent strut, which represented the metal components of the stent structure and coil mass as 3-dimensional (3D) reconstructed images. Impaired stent visualization due to metal artifacts arising from coil materials was minimized through the metal artifact reduction process of cutting outside the region of interest and adjusting the histogram ( Fig. 3). The minimum-to-maximum (m/M) ratio of the deployed stent diameter was measured, except for the flared ends, and the value was calculated to be approximately 0 as the folding by stent kinking was more severe.
Clinical and angiographic outcomes
An IAE was defined as intraoperative in-stent thrombosis or distal emboli, transient ischemic attack (TIA), cerebral infarction, and delayed in-stent stenosis at the follow-up DSA after 1 year. In-stent stenosis was classified as mild (Ōēż33%), moderate (Ōēź33%, <67%), and severe stenosis (Ōēź67%). Hemorrhagic adverse events (HAEs) included intraoperative or delayed rupture of the aneurysm and cerebral hemorrhage. The modified Rankin Scale (mRS) before treatment and at the last clinical follow-up was compared, and mortality was defined as treatment-related death.
Angiographic outcomes were evaluated through immediate postoperative DSA and follow-up DSA after 1 year. Aneurysm occlusion was graded using the modified Raymond-Roy occlusion classification (mRRC) (class I, complete obliteration; class II, persistent opacification of the aneurysm neck; class IIIa, filling of the aneurysm dome within the coil interstices; and class IIIb, contrast filling adjacent to the aneurysm body wall) [ 21] and classified into four categories compared with the immediate degree of mRRC (complete occlusion, mRRC 1 in the follow-up DSA; improvement, decreased mRRC; stabilization, unchanged mRRC; and recanalization, increased contrast filling).
Statistical analysis
The angle measurement for each bend in the carotid siphon was a manual process of converting the 3D structure into a 2D plane and drawing lines along the central axis of the vessel. Three independent reviewers (YSK, SMK, and CHK) measured the angle individually, and the means of the measured values were recorded. The tasks of identifying the ISA and grading the aneurysm occlusion via mRRC were also consensually assessed. Inconsistent results were recorded as negatives.
Categorical variables are presented as frequencies and percentages and were compared using the chi-squared test or Fisher's exact test. Continuous variables are presented as the mean┬▒standard deviation and were compared using an independent t test, Mann-Whitney test, or one-way analysis of variance. Predictive factors of ISA and IAE were determined by logistic regression analysis, and the variables that met the cutoff value of p<0.05 for the univariate analysis were included in the multivariate analysis. Pearson's correlation analysis was used to investigate the relationship between the anterior bend angle and the m/M ratio of the deployed stent diameter. Statistical significance was set at p<0.05. Statistical analyses were performed using R version 4.0.4 (R Development Core Team, Vienna, Austria).
RESULTS
Baseline characteristics
The clinical data of 183 patients with 195 distal ICA aneurysms treated with LVIS SAC depending on the status of stent apposition are summarized in Table 1. All patients had an mRS score of 0 before treatment, and 12 patients underwent SAC for two aneurysms in one LVIS stent. The mean time to follow-up DSA was 372.52┬▒12.69 days, and the mean time to last follow-up was 565.10┬▒242.72 days. The mean angles of the posterior, anterior, and superior bends of the carotid siphon were 87.54┬░┬▒31.64┬░, 18.53┬░┬▒25.76┬░, and 107.98┬░┬▒22.10┬░, respectively, and the anterior bend angle was significantly lower among the three bends ( p<0.001). The anterior bend was divided into 24 angled V-types (13.1%) and 159 non-angled types (86.9%), with 68 C- (37.2%), 79 U- (43.2%), and 12 S-types (6.6%), respectively.
ISA
The ISA did not appear in the superior bend, where the distal flared end was mounted. There were 14 edge malpositions of the fish-mouth shaped proximal flared end in the posterior bend; however, 13 cases were completely corrected using microwire massage. Thus, ISA was found only once in the posterior bend. The 32 trunk malpositions in the anterior bend occurred at a location unrelated to the coil support, which was the proximal portion of the anterior bend away from the aneurysmal neck. They remained ISAs without stent adjustment. There was no case where the coil support was unstable due to the ISA at the position of the aneurysmal neck. Therefore, 33 ISAs (18.0%) were identified. However, no coil loop protrusion across the stent strut was observed. Furthermore, the results did not change at the follow-up DSA.
IAEs and HAEs
There were 27 IAEs (14.8%) in our study ( Table 2). Intraoperative in-stent thrombosis was observed in two cases. Both patients awoke without neurologic deficits through the administration of tirofiban. Thirteen TIAs were observed, including seven visual disturbances or photophobias, three hypoesthesias, two hemipareses, and one sensory aphasia. Four middle cerebral artery territories and one thalamus infarction also occurred. These 18 symptomatic IAEs appeared at a mean postoperative time of 24.31┬▒43.61 days. Three patients were left with permanent deficits. Delayed in-stent stenosis at follow-up DSA occurred in seven cases comprising five mild and two moderate stenoses, all of which were asymptomatic and observed without any intervention. Of the 27 IAEs, five (18.5%) were in a state of ISA. There were two HAEs associated with intraoperative aneurysm ruptures. Further coil insertion was performed as soon as contrast extravasation was observed outside the aneurysm lumen. Fortunately, they were in a good state of stent apposition, and balloon inflation was conducted at the position of the aneurysm neck to stop the bleeding. Both recovered well, with an mRS score of 0 despite subarachnoid hemorrhage. Delayed aneurysm rupture and cerebral hemorrhage were not observed in the present study. Overall, good clinical outcomes with an mRS score of 0-1 were observed in 180 patients (98.4%), and no patients died during the study period.
Immediate and follow-up degree of mRRC
Immediate angiographic outcomes were as follows : mRRC class I of 69.7%, class II of 14.9%, class IIIa of 8.7%, and class IIIb of 6.7%, which improved at follow-up DSA to 90.8% for class I, 5.1% for class II, 1.0% for class IIIa, and 3.1% for class IIIb. Progression to complete obliteration occurred in 21.1% of the patients, and recanalization of the aneurysms was not observed. Delayed stent migration was also not observed on follow-up DSA.
Comparison of CSA and ISA
The CSA and ISA groups were compared to evaluate the stent apposition of the LVIS device. There were no significant differences in the baseline characteristics of sex, age, smoking, alcohol consumption, past medical history, aneurysm configuration and location, ICA diameter, resistance to antiplatelet agents, or specification of stent diameter (p>0.05). The angles of the posterior and anterior bends of the carotid siphon were narrower in the ISA (66.32┬░┬▒35.51┬░, -4.16┬░┬▒25.18┬░) than in the CSA (92.21┬░┬▒28.82┬░, 23.52┬░┬▒23.13┬░) (p<0.001). The ISA occurred more frequently in the angled (V) (41.7%) than in the non-angled type (C, U, and S) (14.5%) (p=0.003). The most frequent ISA in the non-angled type occurred in the S-type (83.3%), followed by the U-type (13.9%) and C-type (2.9%). In the ISA group, we did not intentionally delay the aspirin dose reduction (96.03┬▒37.96 days) or switch from DAPT to SAPT (372.64┬▒13.49 days) compared to the planned regimen. However, treatment outcomes, including IAEs (15.3% vs. 12.1%), HAEs (1.3% vs. 0.0%), aneurysmal complete occlusion (91.3% vs. 88.6%), and recanalization (none of them), did not differ significantly between CSA and ISA (p>0.05).
Predictive factors of ISA
Logistic regression analysis was performed to determine the predictive factors for ISA ( Table 3). The univariate analysis revealed a statistical association between ISA and the following variables : the posterior bend and the U-, S-, and V-type anterior bend in the carotid siphon. The multivariate analysis showed that the S-type (odds ratio, 276.36; 95% confidence interval [CI], 13.65-5595.53; p<0.001) and V-type (OR, 47.70; 95% CI, 6.16-369.13; p<0.001) were independently associated with ISA. However, sex, age, aneurysm configuration and location, ICA diameter, and specification of stent diameter were not associated with ISA. The m/M ratio of the ICA diameter in the range predicted as the stent deployment site was 0.79┬▒0.10, and the m/M ratio of the deployed stent diameter was smaller in ISA (0.37┬▒0.12) than in CSA (0.81┬▒0.09) ( p<0.001). Pearson's correlation analysis showed a positive correlation between the anterior bend angle and the m/M ratio of stent diameter in both angled (correlation coefficient, 0.69; p<0.001) and non-angled types (correlation coefficient, 0.36; p<0.001) ( Fig. 4). The angled type of Ōēż22.8┬░ and the non-angled type of Ōēż-28┬░ caused ISA in all cases.
Predictive factors of IAEs
Logistic regression analysis was also performed to determine the predictive factors for IAEs ( Table 4). Non-responders to clopidogrel (OR, 51.45; 95% CI, 4.66-567.62; p=0.001) had the only independent association with IAEs related to SAC in the multivariate analysis. The %INH for clopidogrel in 27 patients with IAEs (13.78%┬▒14.78%) was significantly lower than that in 156 patients without IAEs (26.82%┬▒20.23%) ( p<0.001). However, sex, age, smoking, alcohol, past medical history, aneurysm configuration and location, bend in the carotid siphon, ICA diameter, aspirin resistance, specification of stent diameter, and ISA were not associated with IAEs.
DISCUSSION
Relationship between ISA and carotid siphon
We measured the angle of each bend in the 3D reconstruction images obtained from rotational angiography since previous studies that evaluated the tortuosity of the ICA from angiographic images of lateral or working projections did not accurately reflect the 3D structure of the carotid siphon [ 25, 26]. Additionally, as an assessment of the stent comprising nitinol wires with low radiopacity on the unsubtracted angiography may not detect the minor ISA of stent struts slightly away from the vessel wall [ 29, 32], all patients were subjected to FDCT with or without a contrast medium, which combines highresolution cone-beam computed tomography with a flat-panel detector of the angiographic system [ 1, 10]. Therefore, the somewhat higher ISA found for 18% of the population may be due to the more detailed inspection and detection conducted in the present study. LVIS Blue demonstrated superior conformability and flexibility when fully expanding in a tortuous carotid siphon compared to the previous LVIS version [ 3, 4, 32]. Edge malposition did not occur at the distal end as finger massage was conducted before being loaded to the microcatheter. It was also resolved in 92.9% of patients at the proximal end through microwire massage after stent release. Trunk malposition was not seen in the posterior bend, despite the significant difference in the angle between the CSA and ISA groups. This was because the posterior bend did not have an acute angle similar to that of the anterior bend. Moreover, the actual stent-subtended angle was gentler than the posterior bend angle when the stent landing zone did not fully cover the posterior bend due to stent length [ 2, 10]. This phenomenon may occur more frequently by stent foreshortening. We focused not only on the angle but also on the morphology of the anterior bend, which is the most troublesome area that causes ISA. It was more vulnerable to ISA in the angled V-type without a round curve that buffers the acute angle of the anterior bend. In the non-angled type with a round curve, the occurrence of ISA was statistically significant only when it reached an S-type <-20┬░. An open-cell design stent may be an alternative for such difficult anatomical structures. However, inward prolapse and increased gap at the inner and outer curvatures of the stent strut could also be obstacles therein [ 12, 30]. Therefore, a comprehensive understanding of the 3D structure of the unique carotid siphon and the manufacturing characteristics of LVIS Blue is the first key to predicting ISA.
Risk and management of ISA
LVIS Blue was durably deployed in all cases during the study period. No coil loop protrusion occurred due to a small cell size, 0.8 mm. Delayed stent migration also did not occur. The angiographic outcomes showed similar or better results when compared to previous studies of LVIS stents, which reported high complete occlusion rates (81.7-93.0%) and low recanalization rates (1.1-2.5%) [ 3, 15, 23, 33]. This is attributed to the flow diversion capacity that changes the hemodynamics of the aneurysm and the progressive endothelialization of the parent artery in contact with stent struts [ 6, 28]. However, despite these excellent results, the tortuous anatomy of the carotid siphon can make the surgeon hesitant to select the LVIS device, designed as a wire-braided stent, since it is difficult to predict the status of stent apposition. This problem inspired the current investigation. Intraprocedural or postprocedural hazards that ISA may cause are as follows : 1) unprotected coil protrusion into the parent vessel by incomplete coverage of the aneurysmal neck; 2) interference with the manipulation of the microcatheter or microwire through the stent lumen (coil-through or jailing technique, reaccess of microcatheter for further coil insertion, or balloon inflation to cease the intraoperative rupture); 3) negative effect of aneurysm occlusion due to obstacle of flow diversion and endothelialization [ 24]; and 4) ischemic complications including thromboembolism and in-stent stenosis [ 12, 33]. The fundamental strategy to prevent or mitigate ISA was the balanced push-pull technique [ 11], and the stent adjustment of microwire massage and balloon angioplasty was minimized due to concerns about device deformity, displacement, or vascular damage. Zhang et al. [ 32] corrected the incidence of 18.7% to 2.5% through active intervention for ISA. However, this caused problems with stent displacement, microwire-induced vessel piercing, and thrombosis. The ischemic complications in the group were too high (39.0%). On the other hand, our clinical outcomes were excellent considering that 27 IAEs (14.8%) included mostly mild TIAs and asymptomatic instent stenosis. Angiographic outcomes were also not affected because all cases of trunk malposition occurred at the proximal portion of the anterior bend of the carotid siphon, away from the aneurysmal neck.
Clopidogrel resistance in SAC
Clinical outcomes were expected to be protected through an appropriate antiplatelet regimen rather than an excessive correction of ISA. Fortunately, there were no differences between the treatment results of CSA and ISA, and only 1.6% of the population had permanent deficits. IAEs were caused by non-responders to clopidogrel, irrespective of ISA. We replaced clopidogrel with cilostazol for this group; however, it failed to alleviate the incidence of IAEs. Although it is well known that clopidogrel resistance is a reliable predictor of IAE-related SAC, the method of evaluating it and the optimal threshold for the PRU or %INH values have not yet been clearly determined, and the optimal antiplatelet dose and coping method for clopidogrel resistance are ongoing issues [ 6, 18]. Alternative drugs with another action mechanism, such as cilostazol, prasugrel, and ticagrelor, have been suggested accordingly [ 8, 9, 13, 17]. However, these drugs need to further demonstrate the improvement of clinical outcomes through multicenter, randomized, and prospective control trials. Our research was limited by the fact that it is a single-center retrospective study that cannot completely exclude potential selection bias. However, we deployed LVIS Blue without the application of any other stent in all patients who were subjected to SAC during the study period. The %INH classification set by our institution is also not an objective criterion that has undergone randomized control trials. In addition, platelet inhibition tests were not subsequently rechecked during the follow-up period after SAC. However, it has recently been proven that %INH is also useful as a tool to predict the thromboembolic risk of coil embolization [ 19]. Therefore, our findings are expected to help support this. Treatment outcomes of ISA in the long-term follow-up after the transition from DAPT to life-long SAPT need to be continuously monitored and investigated. We believe that this study topic would also be interesting when making further investigations into flow-diverting stents.
CONCLUSION
The angled and tortuous anatomical peculiarity of the carotid siphon caused ISA of the LVIS device; however, it was unrelated to clinical and angiographic outcomes, including IAEs, HAEs, aneurysmal complete occlusion, and recanalization. Not responding to clopidogrel, not the ISA, affected IAEs related to SAC. The SAC in the carotid siphon that cannot guarantee CSA should be carefully considered for the use of the stent, design type of device, technical means for deployment, antiplatelet regimen, possible complications, and long-term safety and durability. Further studies are required to identify the appropriate coping methods for antiplatelet regimens in patients with clopidogrel resistance.
Fig.┬Ā1.
A : The carotid siphon was divided into posterior (red), anterior (green), and superior bends (blue). B-E : The anterior bends were categorized into angled (V) and non-angled (C, U, and S) types depending on morphology and measured angles. The angle of each bend was measured at the intersection between two lines traced through the midpoints of the diameters of each straight segment (white dotted lines). B : V-type. C : C-type (Ōēź20┬░). D : U-type (Ōēź-20┬░, <20┬░). E : S-type (<-20┬░). 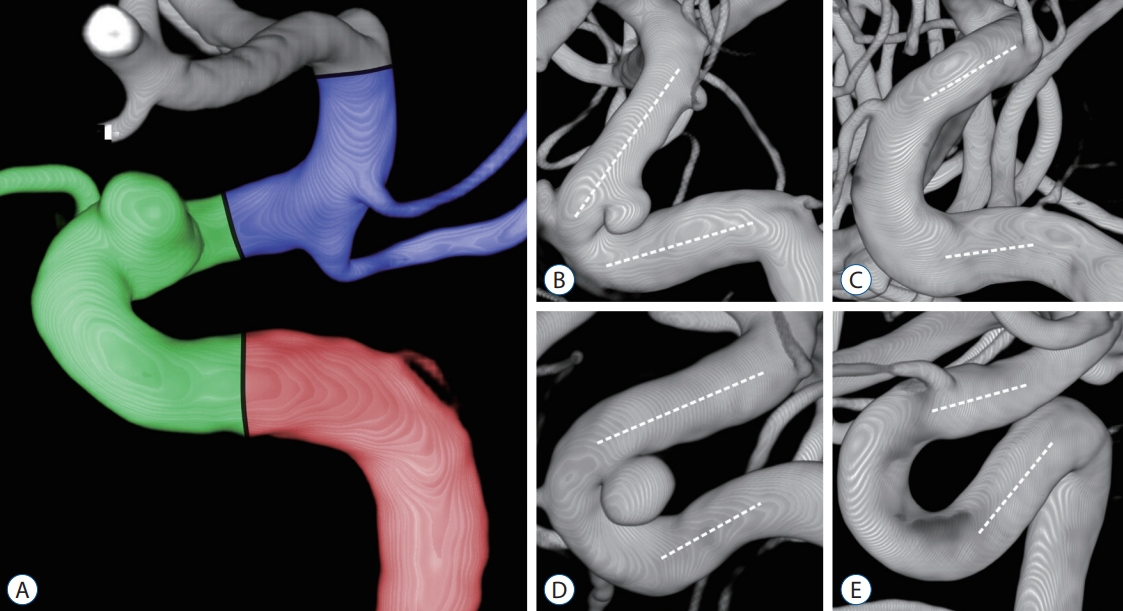
Fig.┬Ā2.
A-C : The push-pull technique was utilized when the two radiopaque strands within the stent body insufficiently contacted the vessel wall. A : Incomplete coverage at the anterior bend of the S-type (-25.5┬░) was identified during stent deployment (white arrow). B : The malpositioned stent was retrieved and redeployed while tension was provided by the push-back of the entire stent system. C : A fully expanded stent is shown on unsubtracted angiography (white arrow). D-F : Balloon angioplasty was only applied in cases of an intraoperative rupture of the aneurysm. D : Contrast leakage outside the lumen was observed due to the superior hypophyseal artery aneurysm rupture (white arrow). E : Balloon angioplasty was applied at the position of the aneurysm neck to stop the bleeding. F : There was no more hemorrhage, and the lesion was completely obliterated. 
Fig.┬Ā3.
A : The status of stent apposition was evaluated by unsubtracted angiography, and the stent struts were in relatively good contact with the arterial wall. B : Trunk malposition was observed in the outer curvature of the anterior bend of the S-type (-45.8┬░) (white arrow). C : Flat-panel detector computed tomography (FDCT) with a contrast medium visualized the stent apposition through a multiplanar reconstruction in the maximal intensity projection mode. D : Trunk malposition was observed in the inner curvature of the anterior bend of the U-type (1.9┬░) (white arrow). E-G : FDCT without a contrast medium represented the metal components of the stent structure and coil mass as 3-dimensional reconstructed images and was taken to identify the degree of stent kinking and coil loop protrusion across the stent strut. H : The stent visualization was relatively well maintained, contrary to the concerns of metal artifacts arising from coil materials. 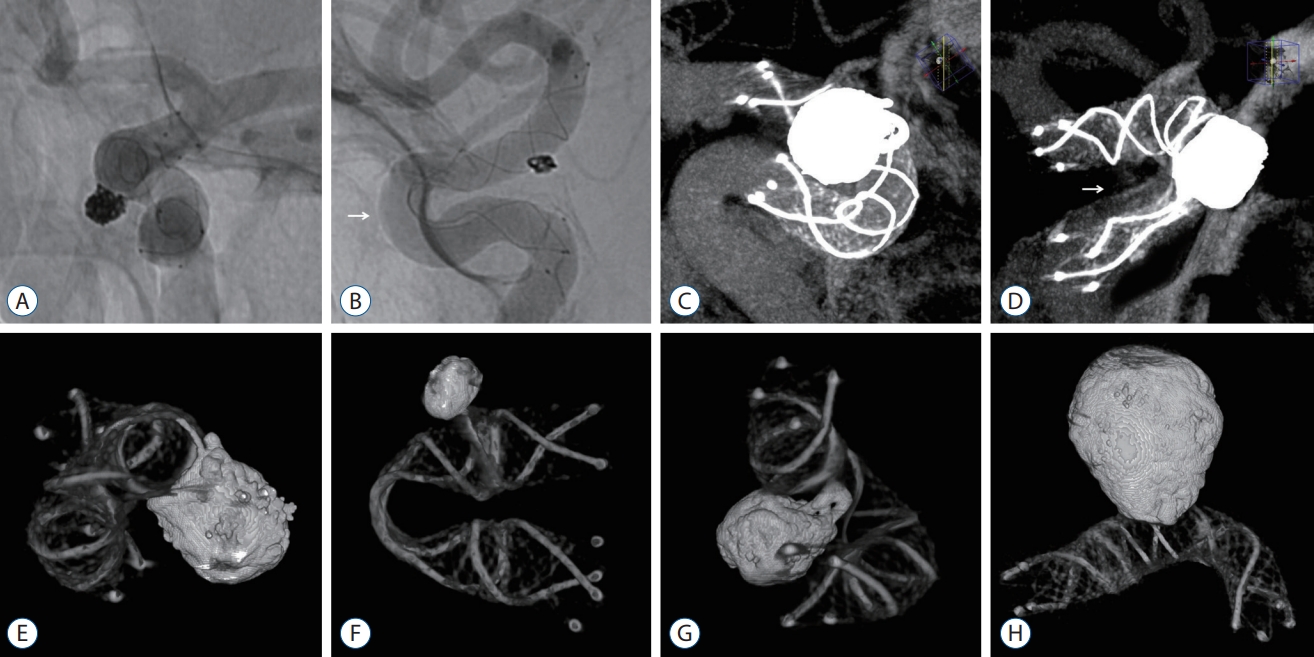
Fig.┬Ā4.
Pearson's correlation analysis showed a positive correlation between the anterior bend angle and the minimum-to-maximum ratio of stent diameter in both (A) angled (correlation coefficient, 0.69; p<0.001) and (B) non-angled types (correlation coefficient, 0.36; p<0.001). CSA : complete stent apposition, ISA : incomplete stent apposition. 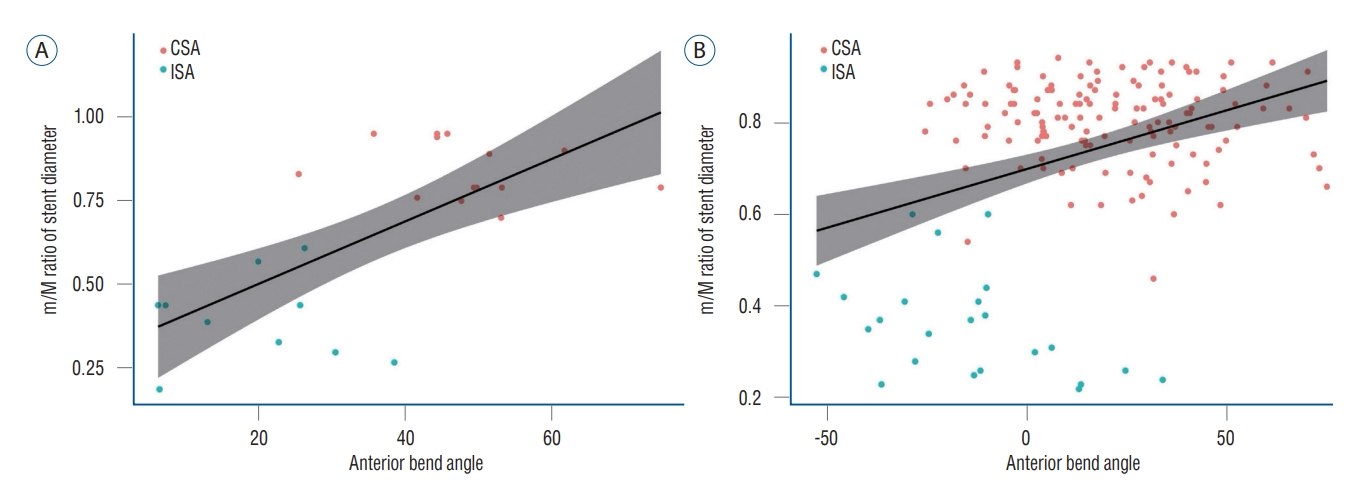
Table┬Ā1.
Baseline characteristics and treatment outcomes of the LVIS Blue stent-assisted coiling of distal internal carotid artery aneurysms depending on the status of stent apposition
|
Variable |
Total |
Stent apposition
|
p-value |
|
CSA |
ISA |
|
Sex, male |
57 (31.1) |
47 (31.3) |
10 (30.3) |
1.000 |
|
Age (years) |
58.10┬▒10.39 |
57.89┬▒10.44 |
59.06┬▒10.26 |
0.556 |
|
Smoking |
20 (10.9) |
16 (10.7) |
4 (12.1) |
0.763 |
|
Alcohol |
48 (26.2) |
40 (26.7) |
8 (24.2) |
0.946 |
|
Hypertension |
50 (27.3) |
39 (26.0) |
11 (33.3) |
0.522 |
|
Diabetes mellitus |
23 (12.6) |
18 (12.0) |
5 (15.2) |
0.572 |
|
Dyslipidemia |
44 (24.0) |
35 (23.3) |
9 (27.3) |
0.799 |
|
Previous hemorrhage |
5 (2.7) |
3 (2.0) |
2 (6.1) |
0.221 |
|
Previous infarction |
6 (3.3) |
6 (4.0) |
0 (0.0) |
0.593 |
|
Prior antiplatelet medication |
17 (9.3) |
16 (10.7) |
1 (3.0) |
0.317 |
|
Aneurysm configuration |
|
|
|
|
|
ŌĆāMaximal diameter (mm) |
5.17┬▒2.04 |
5.18┬▒2.13 |
5.13┬▒1.60 |
0.907 |
|
ŌĆāDome-to-neck ratio |
1.09┬▒0.29 |
1.10┬▒0.29 |
1.05┬▒0.24 |
0.278 |
|
ŌĆāAspect ratio |
0.96┬▒0.32 |
0.97┬▒0.32 |
0.89┬▒0.29 |
0.187 |
|
Aneurysm location : supraclinoid |
34 (17.4) |
30 (18.8) |
4 (11.4) |
0.301 |
|
Carotid siphon : posterior bend (┬║) |
87.54┬▒31.64 |
92.21┬▒28.82 |
66.32┬▒35.51 |
<0.001 |
|
Carotid siphon : anterior bend (┬║) |
18.53┬▒25.76 |
23.52┬▒23.13 |
-4.16┬▒25.18 |
<0.001 |
|
ŌĆāC-type (Ōēź20┬║) |
68 (37.2) |
66 (44.0) |
2 (6.1) |
<0.001 |
|
ŌĆāU-type (-20┬║ to 20┬║) |
79 (43.2) |
68 (45.3) |
11 (33.3) |
|
|
ŌĆāS-type (<-20┬║) |
12 (6.6) |
2 (1.3) |
10 (30.3) |
|
|
ŌĆāV-type |
24 (13.1) |
14 (9.3) |
10 (30.3) |
|
|
Carotid siphon : superior bend (┬║) |
107.98┬▒22.10 |
109.07┬▒21.32 |
103.01┬▒25.13 |
0.205 |
|
ICA diameter (mm) |
|
|
|
|
|
ŌĆāMaximum |
4.68┬▒0.53 |
4.68┬▒0.51 |
4.68┬▒0.61 |
0.938 |
|
ŌĆāMinimum |
3.69┬▒0.47 |
3.69┬▒0.47 |
3.69┬▒0.46 |
0.989 |
|
ŌĆāMaximum-minimum |
0.99┬▒0.51 |
0.99┬▒0.51 |
0.98┬▒0.48 |
0.914 |
|
ŌĆāMinimum/maximum |
0.79┬▒0.10 |
0.79┬▒0.10 |
0.80┬▒0.09 |
0.883 |
|
Aspirin resistance (ARU) |
456.06┬▒70.50 |
455.75┬▒71.36 |
457.45┬▒67.49 |
0.897 |
|
ŌĆāResponder (<550 ARU) |
151 (82.5) |
124 (82.7) |
27 (81.8) |
1.000 |
|
ŌĆāNon-responder (Ōēź550 ARU) |
32 (17.5) |
26 (17.3) |
6 (18.2) |
|
|
Clopidogrel resistance (%INH) |
24.90┬▒20.04 |
23.83┬▒19.56 |
29.73┬▒21.74 |
0.158 |
|
ŌĆāResponder (Ōēź40%) |
41 (22.4) |
31 (20.7) |
10 (30.3) |
0.266 |
|
ŌĆāFewer responder (10% to 40%) |
90 (49.2) |
73 (48.7) |
17 (51.5) |
|
|
ŌĆāNon-responder (<10%) |
52 (28.4) |
46 (30.7) |
6 (18.2) |
|
|
Specification of stent diameter |
|
|
|
|
|
ŌĆā4 mm |
12 (6.6) |
10 (6.7) |
2 (6.1) |
0.754 |
|
ŌĆā4.5 mm |
159 (86.9) |
131 (87.3) |
28 (84.8) |
|
|
ŌĆā5.5 mm |
12 (6.6) |
9 (6.0) |
3 (9.1) |
|
|
m/M ratio of stent diameter |
0.73┬▒0.19 |
0.81┬▒0.09 |
0.37┬▒0.12 |
<0.001 |
|
Aspirin 200 to 100 mg (days) |
96.63┬▒38.43 |
96.77┬▒38.65 |
96.03┬▒37.96 |
0.920 |
|
DAPT to SAPT (day) |
373.52┬▒12.69 |
373.72┬▒12.55 |
372.64┬▒13.49 |
0.674 |
|
Ischemic adverse events |
27 (14.8) |
23 (15.3) |
4 (12.1) |
0.790 |
|
Hemorrhagic adverse events |
2 (1.1) |
2 (1.3) |
0 (0.0) |
1.000 |
|
Aneurysm occlusion |
|
|
|
|
|
ŌĆāComplete occlusion |
177 (90.8) |
146 (91.3) |
31 (88.6) |
0.932 |
|
ŌĆāImprovement |
11 (5.6) |
8 (5.0) |
3 (8.6) |
|
|
ŌĆāStabilization |
7 (3.6) |
6 (3.8) |
1 (2.9) |
|
|
ŌĆāRecanalization |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Table┬Ā2.
Clinical data of patients with ischemic adverse events related to the LVIS Blue stent-assisted coiling of distal internal carotid artery aneurysms
|
No. |
Aneurysm location |
Carotid siphon : anterior bend |
Dyslipidemia |
ARU |
%INH |
Stent apposition |
Ischemic adverse events |
Symptom |
POD |
Last |
Aneury |
|
1 |
OA |
U |
No |
461 |
0 |
CSA |
Intraoperative in-stent thrombosis |
Asymptomatic |
0 |
0 |
C/O |
|
2 |
SHA |
C |
No |
407 |
7 |
CSA |
Intraoperative in-stent thrombosis |
Asymptomatic |
0 |
0 |
C/O |
|
3 |
SHA |
C |
No |
555 |
10 |
CSA |
TIA |
Visual disturbance |
2 |
0 |
C/O |
|
4 |
SHA |
C |
No |
392 |
7 |
CSA |
TIA |
Visual disturbance |
9 |
0 |
C/O |
|
5 |
SHA |
V |
Yes |
511 |
6 |
ISA |
TIA |
Visual disturbance |
16 |
0 |
C/O |
|
6 |
PDW |
U |
No |
405 |
0 |
CSA |
TIA |
Visual disturbance |
26 |
0 |
C/O |
|
7 |
SHA |
U |
No |
432 |
0 |
CSA |
TIA |
Photophobia |
10 |
0 |
C/O |
|
8 |
SHA |
U |
No |
426 |
23 |
CSA |
TIA |
Photophobia |
12 |
0 |
C/O |
|
9 |
OA |
V |
Yes |
392 |
63 |
ISA |
TIA |
Photophobia |
13 |
0 |
Stabilization |
|
10 |
SHA |
C |
No |
413 |
4 |
CSA |
TIA |
Hypoesthesia |
1 |
0 |
C/O |
|
11 |
SHA |
C |
Yes |
398 |
45 |
CSA |
TIA |
Hypoesthesia |
4 |
0 |
C/O |
|
12 |
OA |
U |
Yes |
427 |
20 |
CSA |
TIA |
Hypoesthesia |
7 |
0 |
C/O |
|
13 |
PCoA |
U |
No |
439 |
5 |
CSA |
TIA |
Hemiparesis |
8 |
0 |
C/O |
|
14 |
SHA |
U |
Yes |
487 |
0 |
CSA |
TIA |
Hemiparesis |
23 |
0 |
C/O |
|
15 |
PCoA |
C |
Yes |
427 |
9 |
CSA |
TIA |
Sensory aphasia |
1 |
0 |
C/O |
|
16 |
SHA |
C |
No |
398 |
0 |
CSA |
MCA territory infarction |
Hemiplegia |
7 |
4 |
Improvement |
|
17 |
OA |
U |
Yes |
519 |
5 |
CSA |
MCA territory infarction*
|
Hemiplegia |
8 |
4 |
C/O |
|
18 |
SHA |
S |
Yes |
487 |
0 |
ISA |
MCA territory infarction |
Hemiplegia |
86 |
0 |
C/O |
|
19 |
PDW |
V |
Yes |
567 |
21 |
ISA |
MCA territory infarctionŌĆĀ
|
Hemiplegia |
169 |
0 |
C/O |
|
20 |
SHA |
U |
No |
413 |
22 |
CSA |
Thalamus infarction |
Cognitive impairment |
4 |
2 |
C/O |
|
21 |
SHA |
C |
No |
379 |
23 |
CSA |
Delayed instent stenosis |
Asymptomatic |
361 |
0 |
C/O |
|
22 |
OA |
V |
No |
376 |
15 |
ISA |
Delayed instent stenosis |
Asymptomatic |
371 |
0 |
C/O |
|
23 |
OA |
U |
No |
473 |
28 |
CSA |
Delayed instent stenosis |
Asymptomatic |
371 |
0 |
C/O |
|
24 |
OA |
U |
Yes |
409 |
20 |
CSA |
Delayed instent stenosisŌĆĀ
|
Asymptomatic |
375 |
0 |
Improvement |
|
25 |
PCoA |
C |
No |
401 |
5 |
CSA |
Delayed instent stenosis |
Asymptomatic |
383 |
0 |
Stabilization |
|
26 |
SHA |
C |
No |
391 |
13 |
CSA |
Delayed instent stenosis |
Asymptomatic |
391 |
0 |
C/O |
|
27 |
AchA |
U |
No |
481 |
21 |
CSA |
Delayed instent stenosis |
Asymptomatic |
393 |
0 |
C/O |
Table┬Ā3.
Logistic regression analysis indicating the predictive factors of incomplete stent apposition in the LVIS Blue stent-assisted coiling of distal internal carotid artery aneurysms
|
Variable |
Univariate analysis
|
Multivariate analysis
|
|
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
|
Sex, male |
0.95 (0.42-2.16) |
0.908 |
|
|
|
Age |
1.01 (0.97-1.05) |
0.556 |
|
|
|
Aneurysm configuration |
|
|
|
|
|
ŌĆāMaximal diameter (mm) |
0.98 (0.80-1.19) |
0.824 |
|
|
|
ŌĆāDome-to-neck ratio |
0.36 (0.08-1.65) |
0.188 |
|
|
|
ŌĆāAspect ratio |
0.31 (0.07-1.31) |
0.112 |
|
|
|
Aneurysm location : supraclinoid |
0.60 (0.20-1.85) |
0.374 |
|
|
|
Carotid siphon : posterior bend (┬║) |
0.97 (0.96-0.99) |
<0.001 |
0.99 (0.97-1.02) |
0.594 |
|
Carotid siphon : anterior bend |
Ref. : C-type |
- |
|
|
|
ŌĆāU-type (-20┬║ to 20┬║) |
5.34 (1.14-25.01) |
0.034 |
3.64 (0.58-22.98) |
0.169 |
|
ŌĆāS-type (<-20┬║) |
165.00 (20.83-1307.30) |
<0.001 |
276.36 (13.65-5595.53) |
<0.001 |
|
ŌĆāV-type |
23.57 (4.65-119.59) |
<0.001 |
47.70 (6.16-369.13) |
<0.001 |
|
Carotid siphon : superior bend (┬║) |
0.99 (0.97-1.00) |
0.156 |
|
|
|
ICA diameter (mm) |
|
|
|
|
|
ŌĆāMaximum |
0.97 (0.47-1.99) |
0.930 |
|
|
|
ŌĆāMinimum |
1.01 (0.45-2.26) |
0.989 |
|
|
|
ŌĆāMaximum-minimum |
0.96 (0.46-2.03) |
0.917 |
|
|
|
ŌĆāMinimum/maximum |
1.33 (0.03-68.72) |
0.889 |
|
|
|
Specification of stent diameter |
Ref. : 4 mm |
- |
|
|
|
ŌĆā4.5 mm |
1.07 (0.22-5.15) |
0.934 |
|
|
|
ŌĆā5.5 mm |
1.67 (0.22-12.35) |
0.617 |
|
|
Table┬Ā4.
Logistic regression analysis indicating the predictive factors of ischemic adverse events related to the LVIS Blue stent-assisted coiling of distal internal carotid artery aneurysms
|
Variable |
Univariate analysis
|
Multivariate analysis
|
|
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
|
Sex, male |
1.36 (0.58-3.20) |
0.475 |
|
|
|
Age |
0.98 (0.94-1.02) |
0.300 |
|
|
|
Smoking |
1.52 (0.47-4.96) |
0.486 |
|
|
|
Alcohol |
1.50 (0.62-3.61) |
0.366 |
|
|
|
Hypertension |
1.40 (0.58-3.37) |
0.449 |
|
|
|
Diabetes mellitus |
0.85 (0.23-3.08) |
0.805 |
|
|
|
Dyslipidemia |
2.11 (0.89-5.03) |
0.092 |
|
|
|
Previous hemorrhage |
4.08 (0.65-25.65) |
0.134 |
|
|
|
Previous infarction |
1.16 (0.13-10.35) |
0.893 |
|
|
|
Prior antiplatelet medication |
1.91 (0.57-6.38) |
0.291 |
|
|
|
Aneurysm configuration |
|
|
|
|
|
ŌĆāMaximal diameter (mm) |
1.07 (0.88-1.30) |
0.516 |
|
|
|
ŌĆāDome-to-neck ratio |
0.99 (0.24-4.13) |
0.988 |
|
|
|
ŌĆāAspect ratio |
1.22 (0.37-4.10) |
0.744 |
|
|
|
Aneurysm location : supraclinoid |
1.09 (0.38-3.12) |
0.878 |
|
|
|
Carotid siphon : posterior bend (┬║) |
1.00 (0.98-1.01) |
0.704 |
|
|
|
Carotid siphon : anterior bend |
Ref. : C-type |
|
|
|
|
ŌĆāU-type (-20 ┬║ to 20┬║) |
1.04 (0.42-2.58) |
- 0.935 |
|
|
|
ŌĆāS-type (<-20┬║) |
0.53 (0.06-4.54) |
0.560 |
|
|
|
ŌĆāV-type |
1.16 (0.33-4.11) |
0.818 |
|
|
|
Carotid siphon : superior bend (┬║) |
1.00 (0.98-1.01) |
0.686 |
|
|
|
ICA diameter (mm) |
|
|
|
|
|
ŌĆāMaximum |
1.14 (0.53-2.48) |
0.733 |
|
|
|
ŌĆāMinimum |
0.66 (0.26-1.64) |
0.369 |
|
|
|
ŌĆāMaximum-minimum |
1.61 (0.73-3.58) |
0.240 |
|
|
|
ŌĆāMinimum/maximum |
0.07 (0.00-5.40) |
0.233 |
|
|
|
Aspirin resistance (ARU) |
Ref. : responder |
|
|
|
|
ŌĆāNon-responder (Ōēź550 ARU) |
0.34 (0.08-1.50) |
0.153 |
|
|
|
Clopidogrel resistance (%INH) |
Ref. : responder |
|
|
|
|
ŌĆāFewer responder (10% to 40%) |
2.72 (0.57-12.85) |
0.208 |
|
|
|
ŌĆāNon-responder (<10%) |
7.18 (1.53-33.76) |
0.013 |
51.45 (4.66-567.62) |
0.001 |
|
Specification of stent diameter |
Ref. : 4 mm |
|
|
|
|
ŌĆā4.5 mm |
2.05 (0.25-16.61) |
0.500 |
|
|
|
ŌĆā5.5 mm |
1.00 (0.06-18.09) |
1.000 |
|
|
|
Incomplete stent apposition |
0.76 (0.24-2.37) |
0.638 |
|
|
References
1. Benndorf G, Strother CM, Claus B, Naeini R, Morsi H, Klucznik R, et al : Angiographic CT in cerebrovascular stenting. AJNR Am J Neuroradiol 26 : 1813-1818, 2005   2. Bouillot P, Brina O, Ouared R, Yilmaz H, Farhat M, Erceg G, et al : Geometrical deployment for braided stent. Med Image Anal 30 : 85-94, 2016   3. Cho YD, Sohn CH, Kang HS, Kim JE, Cho WS, Hwang G, et al : Coil embolization of intracranial saccular aneurysms using the low-profile visualized intraluminal support (LVISŌäó) device. Neuroradiology 56 : 543-551, 2014    4. Chung J, Matsuda Y, Nelson J, Keigher K, Lopes DK : A new low-profile visualized intraluminal support (LVIS) device, LVIS Blue: laboratory comparison between old and new LVIS. Neurol Res 40 : 78-85, 2018   6. Dumont TM, Eller JL, Mokin M, Sorkin GC, Levy EI : Advances in endovascular approaches to cerebral aneurysms. Neurosurgery 74 Suppl 1 : S17-S31, 2014   7. Fiorella D, Arthur A, Boulos A, Diaz O, Jabbour P, Pride L, et al : Final results of the US humanitarian device exemption study of the low-profile visualized intraluminal support (LVIS) device. J Neurointerv Surg 8 : 894-897, 2016   8. Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al : Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 120 : 2577-2585, 2009   9. Ha EJ, Cho WS, Kim JE, Cho YD, Choi HH, Kim T, et al : Prophylactic antiplatelet medication in endovascular treatment of intracranial aneurysms: low-dose prasugrel versus clopidogrel. AJNR Am J Neuroradiol 37 : 2060-2065, 2016    10. Heller RS, Malek AM : Parent vessel size and curvature strongly influence risk of incomplete stent apposition in enterprise intracranial aneurysm stent coiling. AJNR Am J Neuroradiol 32 : 1714-1720, 2011    11. Heller RS, Malek AM : Delivery technique plays an important role in determining vessel wall apposition of the enterprise self-expanding intracranial stent. J Neurointerv Surg 3 : 340-343, 2011    12. Heller RS, Miele WR, Do-Dai DD, Malek AM : Crescent sign on magnetic resonance angiography revealing incomplete stent apposition: correlation with diffusion-weighted changes in stent-mediated coil embolization of aneurysms. J Neurosurg 115 : 624-632, 2011   13. Hwang G, Huh W, Lee JS, Villavicencio JB, Villamor RB Jr, Ahn SY, et al : Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: a randomized clinical trial. JAMA Neurol 72 : 764-772, 2015   14. Ikeda H, Otsuka R, Uesaka D, Sano N, Torikoshi S, Hayase M, et al : Effects of acute angle, proximal bending, and distal bending in the deployment vessels on incomplete low-profile visualized intraluminal support (LVIS) expansion: an in vitro study. J Neurointerv Surg 13 : 453-458, 2021   15. Iosif C, Piotin M, Saleme S, Barreau X, Sedat J, Chau Y, et al : Safety and effectiveness of the low profile visualized intraluminal support (LVIS and LVIS Jr) devices in the endovascular treatment of intracranial aneurysms: results of the TRAIL multicenter observational study. J Neurointerv Surg 10 : 675-681, 2018    16. Jankowitz BT, Gross BA, Seshadhri S, Girdhar G, Jadhav A, Jovin TG, et al : Hemodynamic differences between pipeline and coil-adjunctive intracranial stents. J Neurointerv Surg 11 : 908-911, 2019   17. Kim CH, Hwang G, Kwon OK, Ban SP, Chinh ND, Tjahjadi M, et al : P2Y12 reaction units threshold for implementing modified antiplatelet preparation in coil embolization of unruptured aneurysms: a prospective validation study. Radiology 282 : 542-551, 2017   19. Kim YD, Kwon OK, Ban SP, Won YD, Bang JS, Kim T, et al : The inhibition rate estimated using verifynow can help to predict the thromboembolic risk of coil embolization for unruptured intracranial aneurysms. J Neurointerv Surg 14 : 589-592, 2022   20. Koch MJ, Stapleton CJ, Raymond SB, Williams S, Leslie-Mazwi TM, Rabinov JD, et al : LVIS Blue as a low porosity stent and coil adjuvant. J Neurointerv Surg 10 : 682-686, 2018   21. Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, et al : An update to the raymond-roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 7 : 496-502, 2015   22. Matsuda Y, Chung J, Keigher K, Lopes D : A comparison between the new Low-profile Visualized Intraluminal Support (LVIS Blue) stent and the Flow Redirection Endoluminal Device (FRED) in bench-top and cadaver studies. J Neurointerv Surg 10 : 274-278, 2018    23. Mokin M, Primiani CT, Ren Z, Piper K, Fiorella DJ, Rai AT, et al : Stent-assisted coiling of cerebral aneurysms: multi-center analysis of radiographic and clinical outcomes in 659 patients. J Neurointerv Surg 12 : 289-297, 2020   24. Rouchaud A, Ramana C, Brinjikji W, Ding YH, Dai D, Gunderson T, et al : Wall apposition is a key factor for aneurysm occlusion after flow diversion: a histologic evaluation in 41 rabbits. AJNR Am J Neuroradiol 37 : 2087-2091, 2016    25. Waihrich E, Clavel P, Mendes GAC, Iosif C, Moraes Kessler I, Mounayer C : Influence of carotid siphon anatomy on brain aneurysm presentation. AJNR Am J Neuroradiol 38 : 1771-1775, 2017    26. Waihrich E, Clavel P, Mendes G, Iosif C, Kessler IM, Mounayer C : Influence of anatomic changes on the outcomes of carotid siphon aneurysms after deployment of flow-diverter stents. Neurosurgery 83 : 1226-1233, 2018   27. Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S, et al : Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with Enterprise stents and the Pipeline device. J Transl Med 14 : 199, 2016    28. Wanke I, Forsting M : Stents for intracranial wide-necked aneurysms: more than mechanical protection. Neuroradiology 50 : 991-998, 2008    29. Wu P, Ocak PE, Wang D, Ocak U, Xu S, Li Y, et al : Endovascular treatment of ruptured tiny intracranial aneurysms with low-profile visualized intraluminal support device. J Stroke Cerebrovasc Dis 28 : 330-337, 2019   30. Yuki I, Ishibashi T, Dahmani C, Kato N, Ikemura A, Abe Y, et al : Combination of high-resolution cone beam computed tomography and metal artefact reduction software: a new image fusion technique for evaluating intracranial stent apposition after aneurysm treatment. BMJ Case Rep 12 : e2306872019    31. Zhang C, Pu F, Li S, Xie S, Fan Y, Li D : Geometric classification of the carotid siphon: association between geometry and stenoses. Surg Radiol Anat 35 : 385-394, 2013    32. Zhang H, Gao X, Liang H, Ren Y : Incomplete stent apposition of low-profile visualized intraluminal support stents in the treatment of cerebral aneurysms. J Neurointerv Surg 12 : 591-597, 2020   33. Zhang X, Zhong J, Gao H, Xu F, Bambakidis NC : Endovascular treatment of intracranial aneurysms with the LVIS device: a systematic review. J Neurointerv Surg 9 : 553-557, 2017  
|
|




















