Lee, Kim, Jahng, and Park: Value of Perfusion Weighted Magnetic Resonance Imaging in the Diagnosis of Supratentorial Anaplastic Astrocytoma
Abstract
We report perfusion weighted imaging (PWI) findings of nonenhanced anaplastic astrocytoma in a 30-year-old woman. Brain magnetic resonance imaging showed a nonenhanced brain tumor with mild peritumoral edema on the right medial frontal lobe and right genu of corpus callosum, suggesting a low-grade glioma. However, PWI showed increased relative cerebral blood volume, relative cerebral blood flow, and permeability of nonenhanced brain tumor compared with contralateral normal brain parenchyma, suggesting a high-grade glioma. After surgery, final histopathological analysis revealed World Health Organization grade III anaplastic astrocytoma. This case demonstrates the importance of PWI for preoperative evaluation of nonenhanced brain tumors.
Key Words: Anaplastic astrocytoma · MRI · Perfusion weighted MRI.
INTRODUCTION
Glioma is the most common primary tumor of the brain, varying histopathologically from low to high grades 3). Gadolinium-enhanced magnetic resonance imaging (MRI) plays a crucial role in the evaluation of glioma 10). However, the utility of this approach is limited because 15-45% of nonenhanced gliomas are malignant (i.e., anaplastic astrocytoma) and some enhanced gliomas are benign (i.e., pilocytic astrocytoma) 8). Perfusion weighted imaging (PWI) is widely used for preoperative evaluation of glioma, but few studies of the usefulness of PWI solely in nonenhanced glioma have been reported 3,5). Here we describe the cri-tical role of PWI for evaluation of tumor grade in nonenhanced glioma.
CASE REPORT
Patient
A 30-year-old woman who had recently given birth was hospitalized for headache and nausea that developed 24 hours previously. On neurological examination, she had visual disturbance, just before headache onset. Motor and sensory function of the upper and lower extremities were intact. Nonenhanced brain computed tomography (CT) showed a tumor-like lesion with peritumoral edema at the right medial frontal lobe and right genu of corpus callosum. Two days after CT examination upon admission, enhanced brain MRI and PWI were obtained for evaluation of her brain tumor and she was injected with 30 mg prednisolone due to MR contrast allergy.
Imaging acquisition
MRI was performed on a 3T MR system (Philips Medical Systems, Best, The Netherlands) with a 32-channel sensitivity encoding (SENSE) head coil. The order of MRI scans was nonenhanced three-dimensional (3D) sagittal T1-weighted imaging (T1WI), axial fluid attenuated inversion recovery, axial dynamic contrast-enhanced (DCE) PWI, axial and coronal T2-weighted imaging (T2WI), dynamic susceptibility contrast-enhanced (DSC) PWI, and enhanced 3D sagittal T1WI. DCE PWI was performed using a 3D spoiled-gradient recalled echo sequence and DSC PWI was obtained with a 2D axial T2*W gradient echo planar image. The imaging parameters were as follows : for DCE PWI (TR/TE/FOV/voxel size/dynamic scan time/acquisition matrix/reconstruction matrix/acquisition voxel size/reconstruction voxel size/dynamic scan/SENSE factor/number of slices/flip angle=4.4 msec/2.2 msec/210×210 mm2/1.25×1.25×3.5 mm/2.5 sec/168×168/192×192/1.25×1.25×7/1.09×1.09×3.5/106/2.2/23/10°) and DSCPWI (1800 msec/35 msec/220×220 mm2/1.72×1.72×4.5 mm/1.8 sec/128×128/128×128/1.72×1.72×4.5/1.72×1.72×4.5/50/2/24/40°).
Imaging processing
MR perfusion data was post-processed using the NordicICE perfusion module (Nordic ICE; NordicNeuroLab, Bergen, Norway). Normalized all-perfusion maps were obtained and co-registered with enhanced T1WI and T2WI using this software. From DSC PWI, we obtained relative cerebral blood volume (rCBV) and relative cerebral blood flow (rCBF) from regions of maximal solid tumor abnormality on each color map. From DCE PWI, permeability (Ktrans) and area under the curve (AUC) were derived using a pharmacokinetic modeling algorithm in regions of maximal tumor abnormality on each color map. Three regions-of-interest (ROIs) were defined at the solid portion of the tumor on AUC, peritumoral edema on T2WI, and contralateral normal brain. We used three ROIs because we wanted to establish perfusion alterations of the tumor and peritumoral edema compared with normal brain parenchyma.
Results of brain MRI confirmed the presence of a tumor, which had low signal intensity on T1WI, but high signal intensity on T2WI. Gadolinium-enhanced T1WI showed a tumor without enhancement ( Fig. 1). There was a local mass effect on the adjacent right lateral ventricle and a small amount of associated edema ( Fig. 1). Correspondingly high rCBV, rCBF, AUC and increas-ed permeability color values were detected ( Fig. 2, 3). Maximal rCBV and rCBF ratios were also high, at 3.6 and 3.1, respectively. There was a mismatch between the perfusion maps, which suggested high-grade glioma, and enhanced T1WI, which suggested low-grade glioma.
Surgery and histopathology
She underwent frontal craniotomy and subtotal excision of the tumor. The mass tend to have infiltrative nature with poorly delineated margins that grow into the surrounding tissue, making it difficult to completely remove during surgery. It was histopathologically consisted of elongated cells, spongiform foci of astrocytes, large gemitocytes with fibrillar network and microcystic changes. The tumor cells displayed increased cellularity, mitotic activity, distinct nuclear atypia and focal hemorrhage. Tumor cells were immunopositive for glial fibrillary acidic protein. Ki-67 index was approximately 20% in the highest proliferating areas. A diagnosis of anaplastic astrocytoma, World Health Organization grade III, was made. In the two weeks after partial tumor resection, she was treated with adjuvant radiation therapy to right frontal lobe; total dose of 6300 cGy in 30 fractions at 210 cGy/fraction was given from 6th March to 24th April 2012. In April 2012, all radiation therapies were stopped after proving complete remission. She was followed clinically and with serial MRI scans at one month and then six month intervals thereafter. Presently, she has some complications such as hair loss but no evidence of remained tumor.
DISCUSSION
Grading of glioma is of clinical importance because high-grade glioma is usually treated with adjuvant chemo- or radiotherapy after resection, whereas low-grade glioma is not 5). Traditionally, the extent of contrast enhancement has been a marker of malignancy. On gadolinium-enhanced MRI, most high-grade gliomas exhibit moderate to strong enhancement, whereas the low-grade gliomas show minimal or no enhancement 7). However, a broad spectrum of histological types may present as nonenhanced glioma. Thus additional MRI features such as solid tumor heterogeneity, cyst formation, necrosis, mass effect and peritumoral edema, are correlate significantly with malignant behavior 8,10). In addition, the risk of anaplasia increases significantly with patient age 2). However, these factors are not critically correlated with tumor grade. Therefore, in nonenhanced brain tumors, preoperative grade evaluation on conventional brain MRI is often difficult. The two most important factors in determining glioma malignancy are the ability to infiltrate the brain parenchyma and to synthesize and proliferate vascular networks for further growth. Therefore, direct measurement of angiogenesis by activated endothelial cells is a primary criterion for grading of the biological aggressiveness and histology of glioma 5,11). PWI has been used for assessment of tumor vascularity. rCBV and K trans have been shown to correlate reliably with tumor grade and histological tumor microvascularity findings 5,7,11). In our case, we examined DSC and DCE PWI findings of nonenhanced anaplastic astrocytoma. We simultaneously described rCBV (AUC), rCBF, leakage and permeability changes in anaplastic astrocytoma. Although rCBV and permeability have been correlated with glioma, the correlations of three parameters including rCBF with nonenhanced anaplastic astrocytoma determined by both DSC and DCE PWI have not been determined. Furthermore, maximum rCBV in peritumoral edema and normal white matter were also determined in this study. The results indicated the presence of rCBV ratio compared with normal brain parenchyma. Increased peritumoral perfusion may have been due to tumor infiltration. In anaplastic astrocytoma, we hypothesize that peritumoral edema demonstrates not only an altered capillary morphology but also scattered tumor cells. Taken together, the DSC and DCE values of the solid portion of the tumor and peritumoral edema on PWI add more information to that obtained by conventional MRI for differentiation and grading of nonenhanced glioma 6). Gadolinium-enhanced MRI reflects disruption of the blood-brain barrier, and not tumor angiogenesis 2). Formation of new blood vessels is critical for tumor growth, and vascular proliferation of glioma is a diagnostic criterion for malignancy 3). Increased vascular diameter, vessel wall thickness and vessel numbers are common histopathological findings in high-grade glioma, hence, these features should lead to increased blood volume measurements by DSC and DCE MRI 9,11,12). Several studies have demonstrated the relevance of assessment of tumor angiogenesis for diagnosis of glioma and in treatment planning and follow-up 1,9,11). CBV maps can be used to assess tumor neovascularity, which is thought to correlate with tumor grade 1,9). rCBF has been studied as part of the preoperative assessment of tumor grade only rarely compared with rCBV. Shin et al. 11) demonstrated 17 patients with proven glioma and determined rCBF in addition to the rCBV of glioma with promising results. Furthermore, rCBV and rCBF measurement may reduce the sampling error in the histopathological diagnosis of glioma, improving the selection of targets for stereotactic biopsy and facilitating non-invasive differentiation of radiation necrosis from recurrent tumor 9). In addition, perfusion maps may aid early evaluation of therapeutic agents, particularly those aimed at suppressing growth of tumor blood vessels 9). Migration into neighboring cells and tissues is a key feature of glioma which increases as it progresses from low to high grade. Tumor cell extravasation and infiltration is facilitated by extracellular matrix degradation. The resulting breach of the blood-brain barrier is associated with many pathological conditions of the brain 1,4). K trans was found to differentiate low-grade from high-grade glioma. Also, perfusion indices may prove to be important in the assessment of tumor progression, provided the follow-up data are available to determine its correlation with changes in tumor behavior following chemoradiotherapy 1,2,4). In conclusion, combined DCE and DSC PWI can be used to grade nonenhanced brain glioma non-invasively, which may facilitate appropriate treatment planning and management of these patients.
Acknowledgements
A special thank you goes to those who contributed to this paper : Especially Prof. Kim for his valuable comments and sharing his knowledge.
References
1. Awasthi R, Rathore RK, Soni P, Sahoo P, Awasthi A, Husain N, et al : Discriminant analysis to classify glioma grading using dynamic contrast-enhanced MRI and immunohistochemical markers. Neuroradiology 2012, 54 : 205-213,   2. Bruner JM : Neuropathology of malignant gliomas. Semin Oncol 1994, 21 : 126-138,  3. Fan GG, Deng QL, Wu ZH, Guo QY : Usefulness of diffusion/perfusion-weighted MRI in patients with non-enhancing supratentorial brain gliomas : a valuable tool to predict tumour grading? Br J Radiol 2006, 79 : 652-658,   4. Kassner A, Thornhill R : Measuring the integrity of the human blood-brain barrier using magnetic resonance imaging. Methods Mol Biol 2011, 686 : 229-245,   5. Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, et al : Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 2004, 25 : 746-755,   6. Lupo JM, Cha S, Chang SM, Nelson SJ : Dynamic susceptibility-weighted perfusion imaging of high-grade gliomas : characterization of spatial heterogeneity. AJNR Am J Neuroradiol 2005, 26 : 1446-1454,   7. Maia AC Jr, Malheiros SM, da Rocha AJ, da Silva CJ, Gabbai AA, Ferraz FA, et al : MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol 2005, 26 : 777-783,   8. Mihara F, Numaguchi Y, Rothman M, Kristt D, Fiandaca M, Swallow L : Non-enhancing supratentorial malignant astrocytomas : MR features and possible mechanisms. Radiat Med 1995, 13 : 11-17,  9. Petrella JR, Provenzale JM : MR perfusion imaging of the brain : techniques and applications. AJR Am J Roentgenol 2000, 175 : 207-219,   10. Rees J : Advances in magnetic resonance imaging of brain tumours. Curr Opin Neurol 2003, 16 : 643-650,   11. Shin JH, Lee HK, Kwun BD, Kim JS, Kang W, Choi CG, et al : Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas : preliminary results. AJR Am J Roentgenol 2002, 179 : 783-789,   12. Vajkoczy P, Menger MD : Vascular microenvironment in gliomas. J Neurooncol 2000, 50 : 99-108,  
Fig. 1
T2-weighted image (A) shows homogenously hyperintense mass with mild peritumoral edema and contrast-enhanced T1-weighted image (B) shows homogenously hypointense mass without enhancement on the right medial frontal lobe and right genu of corpus callosum. 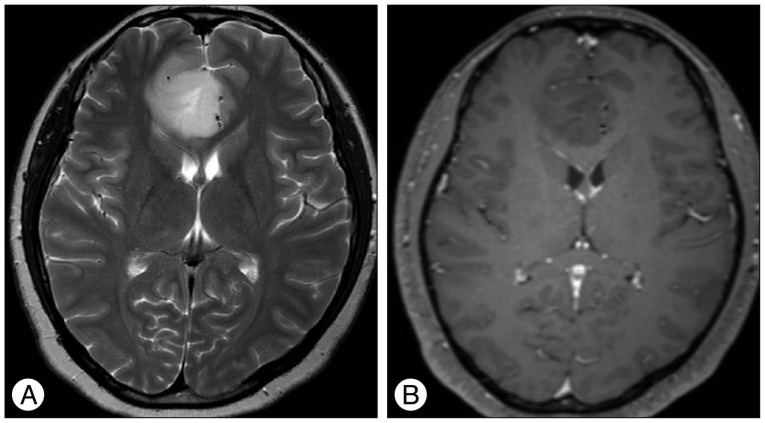
Fig. 2
Perfusion color maps derived from dynamic susceptibility contrast-enhanced perfusion weighted imaging show that the visual increase in the relative cerebral blood volume of 3.6 (A) and relative cerebral blood flow of 2.0 (B). 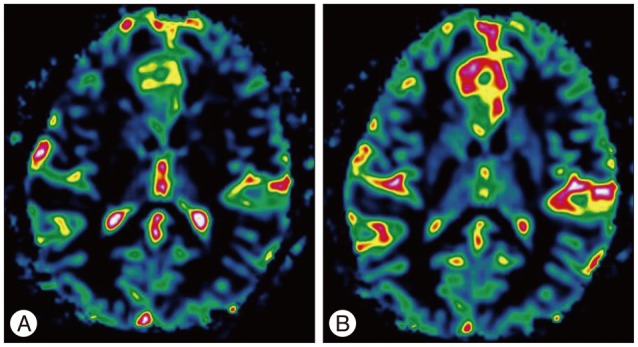
Fig. 3
Perfusion color maps derived from dynamic contrast-enhanced perfusion weighted imaging show that the visual increase in the area under curve (A) and permeability (ktrans) (B). 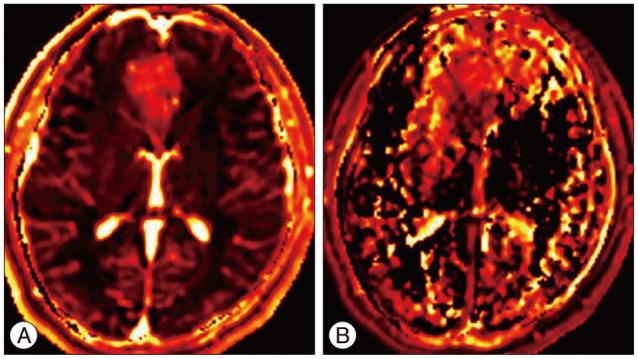
|
|


















