Lee, Lee, Sung, Kim, Kim, and Song: Ki67 Index Is the Most Powerful Factor for Predicting the Recurrence in Atypical Meningioma : Retrospective Analysis of 99 Patients in Two Institutes
Abstract
Objective
The primary objective of this study was to identify predicting factors for local control (LC) of atypical meningioma, and we validated them with comparing the predicting factors for recurrence-free survival (RFS). We also examined the rate of LC after surgical resection with or without adjuvant treatment and RFS.
Methods
Clinical and radiological records of patients with atypical meningiomas diagnosed at two institutes from January 2000 to December 2018 were reviewed retrospectively. Histopathological features were also reviewed using formalin-fixed paraffin embedded samples from pathological archives.
Results
Of the 99 atypical meningiomas eligible for analysis, 36 (36.4%) recurred during the follow-up period (mean, 83.3 months; range, 12-232 months). The rate of 3-year LC and 5-year LC was 80.8% and 74.7%, respectively. The mean time-to-recurrence was 49.4 months (range, 12-150). The mean RFS was 149.3 months (95% confidence interval, 128.8-169.8 months) during the mean follow-up duration of 83.3 months (range, 12-232 months). Multivariate analysis using Cox proportional-hazard regression model showed that the extent of resection (hazard ratio [HR], 4.761; p=0.013), Ki67 index (HR, 8.541; p=0.004), mitotic index (HR, 3.275; p=0.044), and tumor size (HR, 3.228; p=0.041) were independently associated with LC. These factors were also statistically associated with RFS. In terms of radiotherapy after surgical resection, the recurrence was not prevented by immediate radiotherapy because of the strong effect of proliferative index on recurrence.
Conclusion
The present study suggests that the extent of resection, proliferative index (according to Ki67 expression) and mitotic index, and tumor size are associated with recurrence of atypical meningiomas. However, our results should be further validated through prospective and randomized clinical trials to overcome the inborn bias of retrospective nature of the study design.
Key Words: Meningioma, atypical · Recurrence · Growth.
INTRODUCTION
Meningiomas are tumors that arise from the meninges of the brain and spinal cord and account for the most common primary intracranial neoplasms in adults, which in turn account for over a third of primary intracranial neoplasms, with an incident rate of 8.03 per 100000 individuals [ 19]. The 2016 World Health Organization (WHO) classification of tumors of the central nervous system (CNS) categorized meningiomas into three grades [ 16]. Using modern WHO criteria, approximately 70-75% of surgically resected meningiomas are grade I (benign), 20-30% are grade II (atypical), and 1-3% are grade III (anaplastic) [ 16]. Compared to benign meningiomas, atypical or anaplastic tumors have an aggressive clinical behavior, an increased risk of tumor recurrence, and poor prognosis [ 6]. Specifically, atypical meningiomas are more locally aggressive and demonstrate more rapid tumor progression, comparing with benign meningiomas, which are generally slow-growing and have low recurrence rate after gross-total resection (GTR) [ 18, 24]. Literature suggests that atypical meningiomas have a 5-year recurrence rate of approximately 40% in the absence of postoperative radiotherapy (PORT) [ 6, 11]. In fact, previous studies reported an increased risk of recurrence and shorter length of overall survival in atypical meningiomas compared to benign meningiomas; atypical meningioma shows a 7- to 8-fold increased risk of recurrence, and an approximate 2-fold increased risk of death 3-5 years post-diagnosis [ 12, 21]. Despite advances in stereotactic radiosurgery, GTR remains the primary treatment option for meningiomas in most instances, with the goal to achieve complete excision of the dural base of the lesion, akin to Simpson grade 1 resection. Nevertheless, total excision of the meningioma and the dura of origin are not feasible in many instances, or not without incurring unacceptably high rates of postoperative morbidity [ 1]. Therefore, after surgical resection of atypical meningioma, adjuvant treatment modalities should be considered. Immediate PORT is clearly beneficial for malignant meningiomas, while its role for atypical meningiomas is still under debate. Nonetheless, PORT is frequently chosen in cases of atypical meningiomas, despite the absence of clear consensus that this treatment is indicated. There is ongoing debate as to whether atypical meningiomas should receive radiotherapy or whether this treatment should be limited to incompletely resected cases [ 13, 20]. In the clinical practice, it is important for physician to determine whether patients with atypical meningioma should undergo additional treatment after surgical resection, due to their unfavorable and unpredictable prognosis. Several prognostic factors have been reported, such as cellular proliferating factors [ 20], patient age [ 26], tumor location [ 25], preoperative tumor size [ 8], extent of surgical resection [ 14], and early PORT [ 20]. However, these reports are controversial, and the optimum treatment strategy for atypical meningioma remains to be elucidated. In the present study, we retrospectively reviewed the medical records of a large cohort of 99 patients with atypical meningioma who underwent surgical treatment in two institutes. The primary objective of this study was to examine the rate of local control (LC) after surgical resection with or without adjuvant treatment and recurrence-free survival (RFS). We also identified predicting factors for LC of atypical meningioma, and we validated them with comparing the predicting factors for RFS.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Review Boards of Samsung Changwon Hospital (SCMC 2020-03-002) and Dong-A Medical Center (DAUHIRB-20-138). All studies were conducted according to the guidelines of the Declaration of Helsinki for biomedical research. Informed consent was waived due to study’s retrospective nature and minimal hazard to the participants.
Patient’s collection
We conducted a retrospective case study and clinical review of 471 meningioma patients who had been surgically treated at the two individual institutes from January 2000 to December 2018. Patient’s sex and age at the time of surgery, symptoms at diagnosis, tumor location and size, size of peritumoral edema, extent of resection, histological grade, and application of PORT and/or adjuvant chemotherapy, duration of follow-up, recurrence, and survival were retrospectively reviewed for each patient. All atypical meningiomas were newly diagnosed cases, and the following patients were excluded : 1) those with recurrent atypical meningioma after treatment for a previous benign meningioma; 2) those with multiple intracranial meningiomas, because of difficulty to evaluate treatment response; 3) those with spinal meningioma; 4) those who had undergone preoperative radiotherapy against tumors; and 5) those with ≤12 months of follow-up due to follow-up loss.
Histopathological diagnosis
All patients had undergone radical surgery and had a tumor sample for histopathological diagnosis. Among these cases, we selected tumors that met diagnostic criteria for atypical meningioma, as outlined in the 2016 WHO classification of CNS tumors [ 16]. We reviewed again formalin-fixed paraffin embedded (FFPE) samples obtained before 2016 from the pathological archives from two individual institutes to confirm histopathological diagnosis based on the new 2016 WHO diagnostic criteria of atypical meningiomas. According to the new WHO classification of CNS tumors, brain invasion has been added to the existing histological criterion of mitotic count of four or more, which can alone suffice for diagnosing atypical meningioma, WHO grade II [ 16]. Additionally, atypical meningioma can be diagnosed based on the additive criteria of three of the other five histological features : 1) spontaneous necrosis, 2) sheeting (loss of whorling or fascicular architecture), 3) prominent nucleoli, 4) high cellularity, and 5) small cells (tumor clusters with high nuclear:cytoplasmic ratio) [ 16]. The laboratory method for Ki67 analysis as proliferative index and mitosis was performed as followed by the previous protocol [ 14]. Two different neuropathologists, who were blinded to patient clinical and radiological information, reviewed all slides. There was only one discordant case (1.0%) in both reviews of immunoreactivity, which was resolved after discussion.
Neuroradiological findings of atypical meningiomas
Tumor size was defined as the largest tumor diameter rounded to the nearest centimeter on Gadolinium-enhanced T1-weighted magnetic resonance image (MRI) before initial surgery. Peritumoral edema was estimated by the longest distance from the margin of the tumor on fluid-attenuated inversion recovery images. The locations of tumors were divided into convexity (including convexity and parasagittal area) and nonconvexity (including falcine, tentorial, skull base, and intraventricular area) groups. The extent of resection was categorized as Simpson grade 0-5 [ 10]. The extent of resection was estimated not only during the operation itself but also with MRI, which was performed immediately after surgery. Recurrence was defined as the presence of new tumor in patients with a completely resected tumor, as judged in the first postoperative MRI, or as evidence of new growth of an incompletely resected tumor on serial postoperative MRIs compared with immediate postoperative MRIs.
Therapeutic strategies for atypical meningioma
Surgical indications for meningioma were as follows : 1) tumor with neurological symptoms, 2) tumor growing during regular follow-up, 3) tumor size ≥3 cm, 4) tumor requiring a differential diagnosis from other malignancies, and 5) patients’ demand, due to anxiety for tumor growth, even without symptoms. Patients who underwent GTR of atypical meningiomas and did not undergo radiation therapy were closely observed at our institutions. We recommended PORT in all patients with atypical meningiomas that were resected of Simpson grade 3-4 and/or showed high Ki67 index ≥10. Of note, although PORT was commonly applied for the remnant atypical meningioma, strong agreement for this course of action has not been established. In cases of recurrence, reoperation should be considered as the first choice. Some patients, who did not undergo GTR of the tumor, were treated with 3D conformal radiotherapy. Total irradiation dose ranged from 50 to 60 Gy (1.8-2.0 Gy per fraction a day, five fractions a week), depending on the decision from the radiation oncologist. If the patients who were candidates for PORT had been reluctant to radiotherapy, adjunctive chemotherapy was treated after discussion with patients and their family.
Statistical analysis
Differences between subgroups were analyzed using the Student t-test for normally distributed continuous values and the Mann-Whitney U-test for non-normally distributed continuous values. The chi-square test was used to analyze categorical variables. As there is no universal cutoff value for the several clinical factors that predict recurrence of atypical meningiomas, receiver operating characteristic (ROC) curve analysis and sensitivity-specificity analysis was used to define the cutoff value for patient’s age, tumor size, peritumoral edema, Ki67 expression, and Simpson as a predicting factor for the recurrence of atypical meningiomas. Through sensitivity-specificity analysis, the cutoff value (the point at which sensitivity and specificity intersect) was determined for each value, as correlated with recurrence [ 9]. LC and RFS was calculated according to the Kaplan-Meier method, and comparisons between groups were performed using log-rank tests. Variables found to be significantly associated with the LC and RFS of patients with atypical meningiomas in univariate analyses (p-value <0.2) were then subjected to multivariate analyses. Moreover, several additional variables, which have been associated with recurrence of atypical meningioma in the literature and we have been interested in, were also subjected to multivariate analysis. In multivariate analysis, the Cox proportional-hazards regression model was used to assess the independent effects of specific factors on LC and RFS and to define the hazard ratios for significant covariates. Two-sided p-values below 0.05 were considered statistically significant. SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
RESULTS
Clinical and radiological characteristics
From a total of 471 meningioma cases in the defined study period, 105 atypical meningioma patients were eligible for our analysis. Among them, six patients were excluded due to incomplete medical records and/or inadequate features of pathological samples. Eventually, 99 patients (43 males and 56 females) were included in our study. The mean age at diagnosis for these patients was 56.5 years (range, 26.4-87.2) ( Table 1). All 99 patients had undergone radical resection of the tumor. Simpson grade 0-2 was achieved in 61 patients (61.6%), and Simpson grade 3-4 in 38 patients (38.4%) ( Table 1). Out of 61 patients with Simpson grade 0-2, 11 (18.0%) underwent PORT due to high Ki67 index value. Out of 38 patients with Simpson grade 3-4, 17 (44.7%) underwent PORT and 15 (39.5%) were treated with chemotherapy using hydroxyurea. However, the remaining six patients (15.8%) were observed closely without any adjuvant treatment due to their refusal.
Histopathological characteristics
The mean number of mitoses was 8.42 (range, 4-18). The mean Ki67 index was 7.55% (range, 4.0-16.0). Brain invasion was observed in 22 cases (22.2%) ( Table 1). Only three cases, which were diagnosed as WHO grade I meningioma according to the 2007 WHO classification, were updated to WHO grade II atypical meningioma, due to brain invasion features based on the updated WHO classification. No case diagnosed as WHO grade II atypical meningioma according to the 2007 WHO classification changed into different WHO grade tumor classification after review of the FFPE samples.
Favorable factors for recurrence
The mean follow-up time from the date of resection was 83.3 months (range, 12-232). During follow-up, 36 patients (36.4%) presented with recurrence, and all the recurrences occurred at 1 year later after surgery. There was no difference in patient’s age and sex, association with seizure, tumor location, tumor size and peritumoral edema, mean number of mitoses, and PORT between patients with recurrence and those without recurrence. However, tumors with high Ki67 value ( p=0.021) and Simpson grade 3-4 ( p=0.048), and patients who were treated with chemotherapy ( p=0.042) showed more frequent recurrence ( Table 2).
LC
The rate of 3- and 5-year LC were 80.8% and 74.7%, retrospectively ( Fig. 1). In multivariate analysis using logistic regression model, the following factors were independently associated with LC rate; 1) tumor size, 2) surgical resection with Simpson grade, 3) Ki67, and 4) mitotic ( Table 3). However, brain invasion and PORT were not associated with LC rate of atypical meningioma after multi-factor adjustment.
RFS
The mean RFS was 149.3 months (95% confidence interval, 128.8-169.8 months). The cutoff thresholds of the clinical factors determining the association with RFS were calculated with ROC curve analysis ( Table 4). In multivariate analysis using Cox-regression model, the following factors were independently associated with recurrence; 1) surgical resection with Simpson grade, 2) Ki67 index, 3) mitotic index, and 4) tumor size ( Table 5). However, factors which were of interest for the investigators, such as tumor-associated seizure, brain invasion in histopathological feature, PORT, and adjuvant chemotherapy using hydroxyurea were not associated with recurrence of atypical meningiomas ( Table 5). Kaplan-Meier survival analysis for RFS also showed statistically significant differences in the size of the tumor, extent of resection, Ki67 index, and Fig. 2.
Analysis of 36 recurrent cases
The mean time-to-recurrence was 49.4 months (range, 12-150). Most recurrences occurred at the original site of surgery such as the tumor bed (n=7, 19.4%) and tumor margin (n=26, 72.3%). Three patients (8.3%) recurred at a remote site and there was no distant metastasis extracranially ( Table 6). Out of 28 patients who received PORT, 14 (50.0%) recurred, suggesting that PORT could not prevent atypical meningioma from recurrence ( Table 2). Additionally, 10/15 patients (66.7%), who underwent adjuvant chemotherapy, recurred, suggesting that adjuvant chemotherapy could not suppress recurrence of atypical meningioma, either. However, the Ki67 index was much higher in patients who received PORT than in those who did not (9.3% vs. 6.8%, p=0.044). Moreover, the Ki67 index appeared to be higher in patients who underwent adjuvant chemotherapy than in those who did not (8.4% vs. 7.2%, p=0.094). The Ki67 index (hazard ratio of 28.457) was the most powerful factor for predicting recurrence of atypical meningioma compared to any other factors ( Table 5). Out of the 36 patients with recurrent atypical meningiomas, 31 (86.1%) underwent repeated surgical resection, four (11.1%) were treated with salvage radiotherapy, and one (2.8%) underwent salvage chemotherapy alone. The five patients who could not undergo repeated resection had tentorial meningiomas, which were not accessible for surgical resection again. After repeated surgical resection in 31 patients, 12 patients (38.7%) underwent additional salvage radiotherapy, eight patients (29.0%) did salvage chemotherapy, five patients (16.1%) were closely followed up without additional salvage treatment, and another six patients (19.4%) were lost during follow-up. Of the total 36 patients with recurrence, 28 patients were followed up and the mean follow-up period after recurrence was 58.8 (range, 9-199 months). Of these patients, two patients succumbed to malignant transformation of the tumor.
Malignant transformation was found in three (9.7%) atypical meningiomas from WHO grade II to III ( Fig. 3). For other three patients, histopathological diagnosis changed from atypical meningiomas to clear cell or choroid meningioma, which was also classified as WHO grade II meningioma at the repeated surgery.
Analysis of 28 patients who underwent postoperative radiation therapy
The mean time interval between radiation therapy and surgical resection was 29.5 days (range, 25-45). The mean dose of adjuvant radiation therapy was 49.5 Gy (range, 28-54). No patient underwent stereotactic radiosurgery. Although the planned dose of radiation therapy was 54 Gy with 20 fractionations for the adjuvant purpose, five patients did not finished the schedule due to getting worse in general condition.
Between the patients who experienced recurrence and those who did not after adjuvant radiotherapy for atypical meningioma, the sole factor such as proliferative index (Ki67) showed different value ( p=0.043). However, there were no differences in other epidemiological features of patients, characteristics of the tumor, and treatment methods between the patients with recurrence and those without recurrence after adjuvant radiotherapy for atypical meningioma ( Table 6).
DISCUSSION
The purpose of this study was to identify risk factors for the recurrence of atypical meningioma, and our study also examined the rate of LC after surgical resection with or without adjuvant treatment and RFS of atypical meningioma. To the best of our knowledge, the present study provides a unique analysis of the largest cohort with atypical meningiomas, because it is much meaningful that comparative analysis of initial diagnosis with recurrent cases in detail should be performed, which showed the uncommon cases of malignant transition to WHO grade III meningiomas with confirm of histopathologic diagnosis.
Among many predicting factors for recurrence of atypical meningioma, there is no disputing issue that the Ki67 indices should be important predicting factors for disease recurrence [ 2, 4, 5, 14, 15, 20]. In our study, Ki67 index was also found to be the most powerful independent predisposing factor for recurrence of atypical meningioma. It is the known fact that Ki67 and MIB index have related with clinical outcome of meningioma including prognostic and predictive role as well as a high risk related to recurrence in high Ki67 index in meningioma. However, the cutoff value was not firmly established to predict the recurrence of atypical meningioma, especially in those who underwent PORT. The cutoff value of Ki67 index was determined through ROC curve analysis in our study for predicting the recurrence of atypical meningioma. Actually, recurrences occurred in 29/44 cases (65.9%) of atypical meningiomas with Ki67 index ≥7.5%. Out of the 44 patients with atypical meningiomas with Ki67 index ≥7.5%, 20 (45.5%) underwent immediate PORT, and consequently 12/20 patients (60.0%) recurred, suggesting that PORT could not suppress recurrence of atypical meningiomas with high Ki67 index as >7.5%. In contrast, recurrences occurred in 7/55 cases (12.7%) of atypical meningiomas with Ki67 index ≤7.5%. Out of the 55 patients with atypical meningiomas with Ki67 index ≤7.5%, only eight underwent immediate PORT, and consequently two of eight (25.0%) experienced recurrence of disease. Therefore, the proliferative potency may be a good target for treatment of atypical meningiomas. Our analysis showed that the effect of immediate PORT was not demonstrated for the recurrences of atypical meningiomas, which can be much disputable point in presenting study. However, it is difficult for readers to accept the distorted results, because it is true that there was serious selection bias. Patients with unfavorable predicting factors for recurrence such as high proliferative index and/or unsatisfactory resection were candidates for PORT. In fact, 20/44 (45.5%) patients with Ki67 index >7.5% were irradiated immediately after surgery, but only 8/55 (14.5%) patients with Ki67 index ≤7.5% were irradiated immediately after surgery ( p=0.008). In addition, only 11/61 (18.0%) patients with resection of Simpson grade 0-2 were treated with PORT, while 17/38 (44.7%) patients with resection of Simpson grade 3-4 were treated with PORT ( p=0.011). Choi et al. [ 5] also reported that Ki-67 index can be a useful prognostic factor for LC in WHO grade II meningioma, and PORT should be recommended to improve LC in patients with Ki67 index >13%. However, they suggested that PORT should not increase LC for patients with Ki67 index ≤13% [ 5]. Actually, the mean Ki67 index in our study was 7.55% and much lower than 13% in Choi et al. [ 5]'s study. Even in the recurrent cases, the mean Ki67 index was as low as 9.75%, which meant that LC and recurrence in our patients with atypical meningiomas also could not be affected by PORT due to low level of Ki67 index. As the mean Ki67 index for atypical meningiomas ranges from 2.1% to 9.3% in the literatures [ 2, 3, 15], our Ki67 index was not different from the other studies. As mentioned above, there has been not precise cutoff value for predicting the recurrence atypical meningioma, especially in patients who undergo PORT for tumors. It is necessary for investigator to perform the prospective clinical trials in multicentre. In the brief, our results with serious selection bias do not necessarily have an important clinical meaning compared with the literature which has shown the effect of radiotherapy for atypical meningiomas [ 1, 12, 13, 20]. However, it is certain that radiotherapy after surgical resection of atypical meningiomas continues to be controversial. Although most neurosurgeons would not advocate PORT for these meningiomas in case of complete resection, the majority would recommend it in cases of incomplete resection [ 17]. In fact, investigators were puzzled whether PORT may have induced malignant transformation of atypical meningiomas or not. This concern originated from the fact that the three cases with malignant transformation were treated with PORT in our cohorts. No malignant transformation was observed in patients who did not undergo PORT. Indeed, there was a report that 13 of 302 patients (4.3%) with atypical meningioma progressed to WHO grade III meningioma, and all of them were treated with radiotherapy once or more times [ 3]. Randomized and prospective clinical trials are mandatory to define the possibility of radiotherapy’s role on malignant transformation of atypical meningiomas. Our study suggests that several factors, such as extent of resection, Ki67 and mitotic index, and tumor size, are associated with recurrence of atypical meningiomas, and even immediate PORT cannot demonstrate definite effects on reducing recurrence of atypical meningiomas. Also, these results were also compared with other factors from previous literature, but several important limitations must be noted. First of all, the most important limitation is the inherent bias introduced by the retrospective nature of the study. We attempted to reduce this bias by collecting patient data from complete medical and radiological records and by recruiting patients treated using the same protocols. Although multiple investigators, without any prior information for the patients, independently reviewed the pathological slides and radiological images, we cannot clearly claim that no bias originated from this retrospective study. Despite these efforts, however, the conclusions drawn from our study require further validation through prospective and randomized clinical trials. Second, although two different neuropathologists assessed the Ki67 index and mitosis in the samples, we are not certain that the results obtained were correct because the assessment of immunohistochemical staining results is qualitative and often subjective. Reasonable run-to-run reproducibility is essential for proper implementation of these cutoff levels. In addition, threshold levels require adjustment to the sensitivity of the method used. For this reason, we used specificity-sensitivity testing to determine the optimal cutoff level. However, to validate the reproducibility of our immunohistochemical staining method, additional studies are necessary. Third, we did not analyze the molecular and genetic variability, which has an important role on recurrence and malignant transformation. Although we found that the mean Ki67 index increased for recurrent cases from 7.55% to 11.81%, and the mean mitotic index increased for recurrent cases from 8.42 to 10.72, a comprehensive molecular analysis was not performed. It is fact that there are molecular and translational advances in the recent meningioma study. Molecular characterization of meningioma has identified genetic biomarkers that can predict tumor behavior including malignant transformation. Only a few genetic changes are known to classify >85% of all meningioma and clinical trials using targeted therapy to genetic subtypes of meningioma are under way [ 23]. Among several specific molecular genetic biomarkers, such as PI3K/AKT pathway, Hedgehog pathway, and immune check point system, neurofibromatosis 2 (NF2) has been reported as a high incidence in patients with WHO grade II and III meningiomas [ 7, 22, 23]. Perry et al. [ 22] also noted a higher incidence of atypical and malignant meningiomas in patients with NF2 mutation. Translational analysis for molecular and genetic markers are essential for determining the biologic changes of tumor conditions. Finally, despite the presenting study included relatively larger number of patients with infrequent brain tumors in multicenter, our study had not showed strongly novel findings in the field of study on atypical meningioma. Actually, our analysis was dealing several discussing points, such as role of Ki67 index in predicting clinical outcome, efficacy of PORT in atypical meningioma, malignant transformation of grade II meningioma into grade III meningioma, and prognostic factors in atypical meningioma patients. Further comprehensive study is essential to establish concrete concepts about management of atypical meningioma.
CONCLUSION
In this study, we investigated the LC and RFS of atypical meningioma after surgical resection, and identified the associated predicting factors. We found that the Ki67 and mitotic index, extent of resection, and tumor size are associated with the recurrence of atypical meningiomas. Especially, as the proliferative index is a powerful independent predictor for recurrence, the effectiveness of adjuvant treatment including radiotherapy has not been demonstrated against the recurrence of atypical meningioma with a high proliferative index. However, our results require further validation through prospective and randomized clinical trials.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (The Ministry of Science and ICT) (Grant No. NRF 2019R 1F1A 1054681). This paper was also financially supported by Sungkyun Research Fund, Sungkyunkwan University (2016) and Samsung Changwon Hospital Research Fund (2020).
The authors would like to thank Young Min Kim, M.D. and Mi-Ok Sunwoo, M.D. (Department of Radiology, Samsung Changwon Hospital), and Sun Sup Choi, M.D. (Department of Radiology, Dong-A University Medical Center) for reviewing neuroradiological images; Young Wook Kim, M.D. (Department of Biostatistics, Samsung Changwon Hospital) for assistance with statistical analysis, and Tae Gyu Kim, M.D. (Department of Radiation Oncology, Samsung Changwon Hospital), and Young Min Choi, M.D. (Department of Radiation Oncology, Dong-A University Medical Center) for applying the radiotherapy detailed in this work.
Fig. 1.
Local control rate of atypical meningioma after surgical resection with or without following adjuvant treatment. The 3- and 5-year local control rate is 80.8% and 74.7%, respectively. 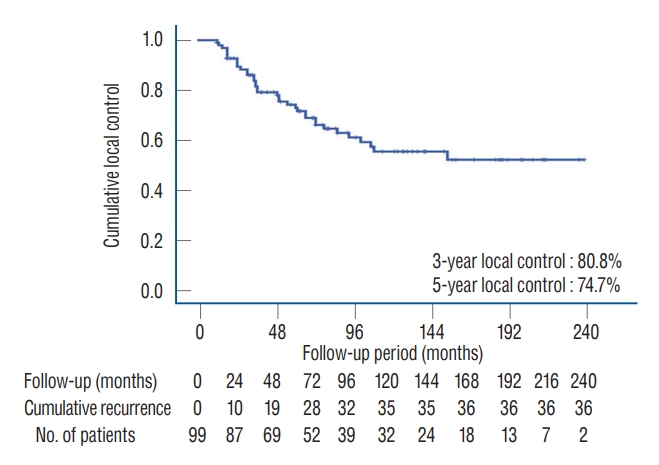
Fig. 2.
Recurrence-free survival curves for patients with atypical meningiomas by Kaplan-Meir survival curve analysis. A : Tumor size (≤4 cm vs. >4 cm). B : The extent of resection (Simpson grade 0-2 vs. 3-4). C : Ki67 (≤7.5% vs. >7.5%). D : Mitotic index (≤8.5 vs. >8.5). E : Immediate postoperative radiotherapy (yes vs. no). F : Adjuvant chemotherapy using hydroxyurea (yes vs. no). 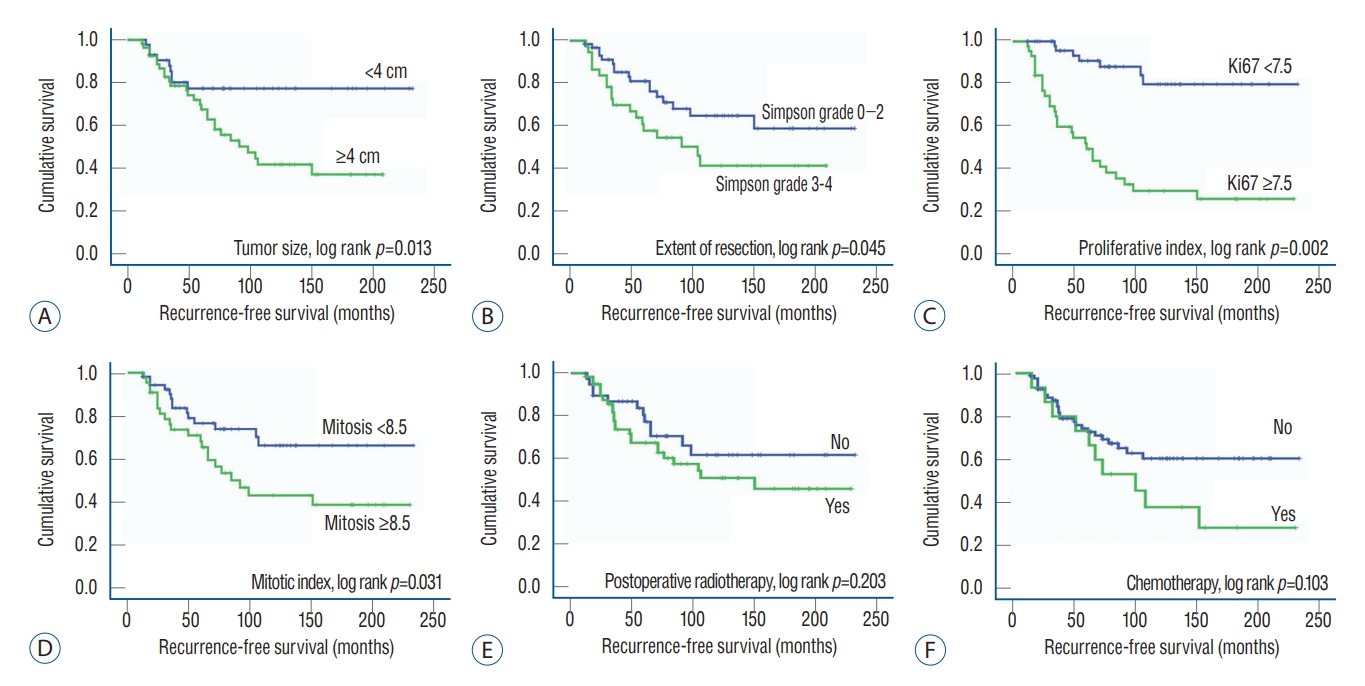
Fig. 3.
Illustration of the cases with malignant transformation (Hematoxylin and Eosin staining, ×40). A : An atypical meningioma of 53-year-old male on the parasagittal area recurred at 72 months after initial surgery, and malignant transformation with marked cellular and nuclear pleomorphism was confirmed. B : An atypical meningioma of 59-year-old female on the falcine area recurred at 82 months after initial surgery, and malignant transformation with frank anaplasia with sarcomatoid appearance was confirmed. C : An atypical meningioma of 55-year-old female on the convexity area was recurred at 34 months after initial surgery, and malignant transformation with the presence of rhabdoid morphology was confirmed. 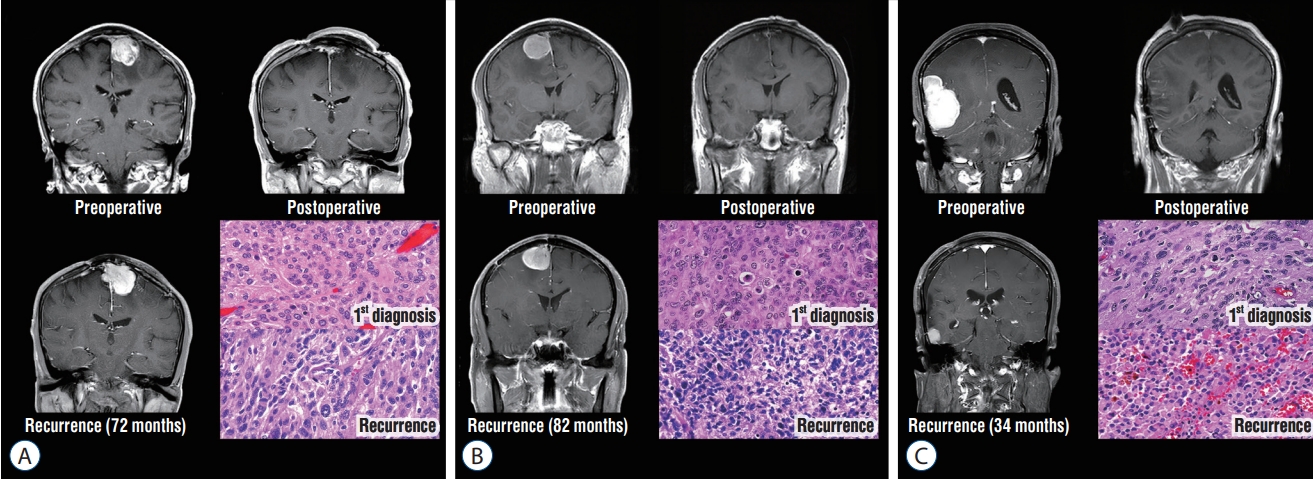
Table 1.
Clinical and radiological characteristics of the 99 patients with atypical meningiomas
|
Factor |
Value |
|
Age (years) |
56.5 (26.4-87.2) |
|
Gender |
|
|
Male |
43 (43.4) |
|
Female |
56 (56.6) |
|
Chief complication |
|
|
Headache |
45 (45.5) |
|
Seizure |
28 (28.3) |
|
Focal neurological deficit |
11 (11.1) |
|
Altered mentation |
6 (6.0) |
|
None |
9 (9.1) |
|
Tumor location |
|
|
Convexity & parasagittal |
50 (50.5) |
|
Falx |
21 (21.2) |
|
Skull base |
13 (13.1) |
|
Tentorial |
8 (8.1) |
|
Intraventricular |
8 (8.1) |
|
Maximal diameter in T1WI with Gd enhancement (cm) |
4.21 (2.05-8.32) |
|
Size of peritumoral edema in T2WI (cm) |
1.87 (0.00-5.62) |
|
Extent of surgical resection |
|
|
Simpson grade 0 |
8 (8.1) |
|
Simpson grade 1 |
34 (34.3) |
|
Simpson grade 2 |
19 (19.2) |
|
Simpson grade 3 |
33 (33.3) |
|
Simpson grade 4 |
5 (5.1) |
|
Value of Ki67 (%) |
7.55 (4-16) |
|
Number of mitosis |
8.42 (4-18) |
|
Brain invasion |
|
|
Yes |
22 (22.2) |
|
No |
77 (77.8) |
|
Postoperative radiotherapy |
|
|
Yes |
28 (28.3) |
|
No |
71 (71.7) |
|
Postoperative chemotherapy |
|
|
Yes |
15 (15.2) |
|
No |
84 (84.8) |
|
Recurrence |
|
|
Yes |
36 (36.4) |
|
No |
63 (63.6) |
Table 2.
Comparative data of the clinical and radiological characteristics in the patients with recurrence versus without recurrence of atypical meningiomas (n=99)
|
Factor |
Recurrence (+) (n=36) |
Recurrence (-) (n=63) |
p-value |
|
Age (years) |
56.3 (32.7-75.5) |
56.8 (26.4-87.2) |
0.984 |
|
Gender |
|
|
0.162 |
|
Male |
13 (36.1) |
30 (47.6) |
|
|
Female |
23 (63.9) |
33 (52.4) |
|
|
Seizure |
|
|
0.251 |
|
Yes |
8 (22.2) |
20 (31.7) |
|
|
No |
28 (77.8) |
43 (68.3) |
|
|
Tumor location |
|
|
0.802 |
|
Convexity |
15 (41.7) |
35 (55.5) |
|
|
Non-convexity |
21 (58.3) |
28 (44.5) |
|
|
Maximal diameter in T1WI with Gd enhancement (cm) |
4.55 (2.63-8.32) |
4.02 (2.05-6.32) |
0.227 |
|
Size of peritumoral edema in T2WI (cm) |
2.51 (0.50-5.62) |
1.50 (0.00-4.55) |
0.122 |
|
Extent of surgical resection |
|
|
0.048 |
|
Simpson grade 0 |
2 (5.6) |
6 (9.5) |
|
|
Simpson grade 1 |
9 (25.0) |
25 (39.7) |
|
|
Simpson grade 2 |
6 (16.7) |
13 (20.6) |
|
|
Simpson grade 3 |
17 (47.1) |
16 (25.4) |
|
|
Simpson grade 4 |
2 (5.6) |
3 (4.8) |
|
|
Value of Ki67 (%) |
9.75 (4-16) |
5.70 (4-12) |
0.021 |
|
Mitotic index |
10.2 (4-18) |
7.40 (4-18) |
0.114 |
|
Brain invasion |
|
|
|
|
Yes |
10 (27.7) |
12 (19.0) |
0.349 |
|
No |
26 (72.3) |
51 (81.0) |
|
|
Postoperative radiotherapy |
|
|
0.074 |
|
Yes |
14 (38.8) |
14 (22.2) |
|
|
No |
22 (61.2) |
49 (77.8) |
|
|
Postoperative chemotherapy |
|
|
0.042 |
|
Yes |
10 (27.8) |
5 (7.9) |
|
|
No |
26 (72.2) |
58 (92.1) |
|
Table 3.
Factors associated with local control rate in 99 patients with atypical meningioma, according to the clinical and radiological characteristics
|
Variable |
3-year local control |
Univariate analysis
|
Multivariate analysis
|
|
Hazard ratio (95% CI) |
p-value |
Hazard ratio (95% CI) |
p-value |
|
Age |
|
|
|
|
|
|
≤55 years |
34/39 (87.2) |
1.429 (0.851-2.008) |
0.546 |
N.A. |
|
|
>55 years |
46/60 (76.7) |
1.000 |
|
|
|
|
Gender |
|
|
|
|
|
|
Male |
38/43 (88.4) |
1.388 (0.764-2.012) |
0.615 |
N.A. |
|
|
Female |
42/56 (75.0) |
1.000 |
|
|
|
|
Seizure |
|
|
|
|
|
|
Yes |
23/28 (82.1) |
1.079 (0.462-1.696) |
0.878 |
N.A. |
|
|
No |
57/71 (80.3) |
1.000 |
|
|
|
|
Location |
|
|
|
|
|
|
Non-convexity |
43/49 (87.8) |
1.517 (0.884-2.149) |
0.443 |
N.A. |
|
|
Convexity |
37/50 (74.0) |
1.000 |
|
|
|
|
Tumor size |
|
|
|
|
|
|
≤4 cm |
40/43 (93.0) |
2.564 (1.436-3.692) |
0.047 |
3.228 (1.725-4.731) |
0.041 |
|
>4 cm |
40/56 (71.4) |
1.000 |
|
1.000 |
|
|
Peritumoral edema |
|
|
|
|
|
|
≤2 cm |
47/55 (85.5) |
1.852 (0.947-2.757) |
0.316 |
N.A |
|
|
>2 cm |
33/44 (73.3) |
1.000 |
|
|
|
|
Brain invasion |
|
|
|
|
|
|
Absence |
65/77 (84.4) |
2.219 (1.272-3.166) |
0.108 |
2.184 (0.989-3.379) |
0.052 |
|
Presence |
15/22 (68.2) |
1.000 |
|
1.000 |
|
|
Extent of resection |
|
|
|
|
|
|
SG 0-2 |
53/61 (86.9) |
2.448 (1.206-3.689) |
0.073 |
4.761 (2.945-6.577) |
0.013 |
|
SG 3-4 |
27/38 (71.1) |
1.000 |
|
1.000 |
|
|
Ki67 index |
|
|
|
|
|
|
≤7.5% |
53/55 (96.4) |
4.981 (2.614-7.348) |
0.018 |
8.541 (4.074-13.088) |
0.004 |
|
>7.5% |
27/44 (61.4) |
1.000 |
|
1.000 |
|
|
Mitotic index |
|
|
|
|
|
|
≤8.5 |
46/54 (85.2) |
1.957 (0.992-2.921) |
0.134 |
3.275 (1.543-5.007) |
0.044 |
|
>8.5 |
34/45 (75.6) |
1.000 |
|
1.000 |
|
|
Postoperative RTx |
|
|
|
|
|
|
No |
62/71 (87.3) |
2.314 (0.948-3.679) |
0.092 |
1.816 (0.801-2.831) |
0.129 |
|
Yes |
18/28 (64.3) |
1.000 |
|
1.000 |
|
|
Postoperative CTx |
|
|
|
|
|
|
Yes |
68/84 (81.0) |
1.066 (0.385-1.747) |
0.910 |
N.A. |
|
|
No |
12/15 (80.0) |
1.000 |
|
|
|
Table 4.
Results of ROC curve analysis and sensitivity-specificity analysis of certain factors with continuous value determining cut-off value
|
Value |
AUC in ROC curve |
Cut-off value |
Sensitivity |
Specificity |
|
Age (years) |
56.6±22.0 |
0.662 |
55 |
0.647 |
0.690 |
|
Tumor size (cm) |
4.21±2.08 |
0.716 |
4.0 |
0.659 |
0.772 |
|
Size of peritumoral edema (cm) |
1.87±1.35 |
0.659 |
2.0 |
0.715 |
0.616 |
|
Value of Ki67 (%) |
7.55±2.62 |
0.821 |
7.5 |
0.774 |
0.888 |
|
Mitotic index |
8.42±3.21 |
0.740 |
8.5 |
0.699 |
0.773 |
|
Simpson grade |
1.93±1.02 |
0.804 |
2.0 |
0.782 |
0.831 |
Table 5.
Factors associated with RFS in 99 patients with atypical meningioma according to the clinical and radiological characteristics
|
Variable |
RFS (months) |
Univariate analysis
|
Multivariate analysis
|
|
Hazard ratio (95% CI) |
p-value |
Hazard ratio (95% CI) |
p-value |
|
Age |
|
|
|
|
|
|
≤55 years |
163.8±15.9 |
1.238 (0.889-1.587) |
0.425 |
N.A. |
|
|
>55 years |
137.4±13.5 |
1.000 |
|
|
|
|
Gender |
|
|
|
|
|
|
Male |
163.3±14.7 |
1.711 (0.926-2.496) |
0.237 |
N.A. |
|
|
Female |
136.4±14.3 |
1.000 |
|
|
|
|
Seizure |
|
|
|
|
|
|
No |
151.4±17.4 |
1.135 (0.448-1.722) |
0.651 |
1.355 (0.647-2.063) |
0.479 |
|
Yes |
144.7±12.1 |
1.000 |
|
1.000 |
|
|
Location |
|
|
|
|
|
|
Convexity |
157.5±14.5 |
1.105 (0.519-1.691) |
0.843 |
N.A. |
|
|
Non-convexity |
129.0±13.2 |
1.000 |
|
|
|
|
Tumor size |
|
|
|
|
|
|
≤4 cm |
186.2±13.5 |
6.115 (3.041-9.187) |
0.032 |
7.543 (4.682-10.404) |
0.011 |
|
>4 cm |
117.2±11.5 |
1.000 |
|
1.000 |
|
|
Peritumoral edema |
|
|
|
|
|
|
≤2 cm |
157.1±13.7 |
1.099 (0.608-1.589) |
0.756 |
N.A. |
|
|
>2 cm |
128.2±13.8 |
1.000 |
|
|
|
|
Brain invasion |
|
|
|
|
|
|
Presence |
155.0±11.6 |
2.716 (0.943-4.489) |
0.082 |
2.050 (0.822-3.278) |
0.152 |
|
Absence |
108.8±19.3 |
1.000 |
|
1.000 |
|
|
Extent of resection |
|
|
|
|
|
|
SG 0-2 |
164.5±13.2 |
3.868 (2.487-7.736) |
0.041 |
5.724 (3.245-8.023) |
0.031 |
|
SG 3-4 |
116.7±14.4 |
1.000 |
|
1.000 |
|
|
Ki67 |
|
|
|
|
|
|
≤7.5% |
200.0±11.0 |
27.626 (19.543-35.709) |
<0.001 |
28.457 (21.286-35.628) |
<0.001 |
|
>7.5% |
96.8±13.6 |
1.000 |
|
1.000 |
|
|
Mitotic index |
|
|
|
|
|
|
≤8.5 |
172.0±13.4 |
4.643 (2.902-6.384) |
0.031 |
3.441 (1.296-5.586) |
0.049 |
|
>8.5 |
124.4±14.8 |
1.000 |
|
1.000 |
|
|
Postoperative RTx |
|
|
|
|
|
|
No |
161.4±11.7 |
2.056 (0.955-3.157) |
0.152 |
1.214 (0.786-1.642) |
0.203 |
|
Yes |
128.6±16.0 |
1.000 |
|
1.000 |
|
|
Postoperative CTx |
|
|
|
|
|
|
Yes |
159.6±11.4 |
2.661 (0.993-4.328) |
0.067 |
3.027 (0.984-5.071) |
0.058 |
|
No |
116.1±21.2 |
1.000 |
|
1.000 |
|
Table 6.
Subgroup analysis of patients who underwent immediate radiotherapy after surgical resection of atypical meningioma (n=28)
|
Factor |
Recurrence (+) (n=18) |
Recurrence (-) (n=10) |
p-value |
|
Age (years) |
55.6 (34.6-75.5) |
58.8 (40.8-72.4) |
0.425 |
|
Gender |
|
|
0.066 |
|
Male |
6 (33.3) |
6 (60.0) |
|
|
Female |
12 (66.6) |
4 (40.0) |
|
|
Tumor location |
|
|
0.537 |
|
Convexity |
7 (38.9) |
3 (30.0) |
|
|
Non-convexity |
11 (61.1) |
7 (70.0) |
|
|
Maximal diameter in T1WI with Gd enhancement (cm) |
4.33 (2.03-6.26) |
4.20 (2.27-5.28) |
0.708 |
|
Size of peritumoral edema in T2WI (cm) |
1.84 (0.50-3.21) |
1.95 (0.00-3.22) |
0.614 |
|
Extent of surgical resection |
|
|
0.387 |
|
Simpson grade 0 |
1 (5.6) |
1 (10.0) |
|
|
Simpson grade 1 |
1 (5.6) |
3 (30.0) |
|
|
Simpson grade 2 |
6 (33.3) |
1 (10.0) |
|
|
Simpson grade 3 |
8 (44.4) |
4 (40.0) |
|
|
Simpson grade 4 |
2 (11.1) |
1 (10.0) |
|
|
Brain invasion |
|
|
|
|
Yes |
5 (27.8) |
3 (30.0) |
0.628 |
|
No |
13 (72.2) |
7 (70.0) |
|
|
Value of Ki67 (%) |
10.4 (4-15) |
7.7 (4-12) |
0.043 |
|
Mitotic index |
10.5 (6-18) |
9.0 (5-15) |
0.351 |
|
Timing of radiotherapy after surgery (days) |
29.8 (25-42) |
28.9 (25-45) |
0.872 |
|
Dose of radiotherapy (Gy) |
49.5 (28-54) |
49.6 (34-54) |
0.991 |
|
Additional chemotherapy |
|
|
0.714 |
|
Yes |
5 (27.8) |
1 (10.0) |
|
|
No |
13 (72.2) |
9 (90.0) |
|
References
1. Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, et al : Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64 : 56-60, 2009    2. Champeaux C, Dunn L : World health organization grade II meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg 89 : 1801-186, 2016  3. Champeaux C, Houston D, Dunn L : Atypical meningioma. a study on recurrence and disease-specific survival. Neurochirurgie 63 : 273-281, 2017   4. Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L : WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neurooncol 129 : 337-345, 2016    5. Choi Y, Lim DH, Yu JI, Jo K, Nam DH, Seol HJ, et al : Prognostic value of KI-67 labeling index and postoperative radiotherapy in WHO grade II meningioma. Am J Clin Oncol 41 : 18-23, 2018   6. Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, et al : The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus 30 : E6, 2011  7. Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al : Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339 : 1077-1080, 2013    8. Detti B, Scoccianti S, Di Cataldo V, Monteleone E, Cipressi S, Bordi L, et al : Atypical and malignant meningioma: outcome and prognostic factors in 68 irradiated patients. J Neurooncol 115 : 421-427, 2013    9. Eng J : Receiver operating characteristic analysis: a primer. Acad Radiol 12 : 909-916, 2005  10. Gousias K, Schramm J, Simon M : The simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg 125 : 551-560, 2016   11. Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, et al : Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117 : 1272-1278, 2011    12. Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, et al : Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol 16 : 628-636, 2014    13. Kim M, Cho YH, Kim JH, Kim CJ, Roh SW, Kwon DH : Role of gamma knife radiosurgery for recurrent or residual World Health Organization grade II and III intracranial meningiomas. Br J Neurosurg 34 : 239-245, 2020   14. Kim MS, Kim KH, Lee EH, Lee YM, Lee SH, Kim HD, et al : Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J Neurosurg 121 : 1189-1200, 2014   15. Klinger DR, Flores BC, Lewis JJ, Hatanpaa K, Choe K, Mickey B, et al : Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg 84 : 839-845, 2015   16. Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al : The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131 : 803-820, 2016    17. Marcus HJ, Price SJ, Wilby M, Santarius T, Kirollos RW : Radiotherapy as an adjuvant in the management of intracranial meningiomas: are we practising evidence-based medicine? Br J Neurosurg 22 : 520-528, 2008   18. Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, et al : Meningioma. Crit Rev Oncol Hematol 67 : 153-157, 2008   19. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al : CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united states in 2012-2016. Neuro Oncol 21( Suppl 5):v1-v100, 2019     20. Park HJ, Kang HC, Kim IH, Park SH, Kim DG, Park CK, et al : The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol 115 : 241-247, 2013    21. Perry A : Unmasking the secrets of meningioma: a slow but rewarding journey. Surg Neurol 61 : 171-173, 2004   22. Perry A, Giannini C, Raghavan R, Scheithauer BW, Banerjee R, Margraf L, et al : Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol 60 : 994-1003, 2001   23. Proctor DT, Ramachandran S, Lama S, Sutherland GR : Towards molecular classification of meningioma: evolving treatment and diagnostic paradigms. World Neurosurg 119 : 366-373, 2018   24. Tanzler E, Morris CG, Kirwan JM, Amdur RJ, Mendenhall WM : Outcomes of WHO grade I meningiomas receiving definitive or postoperative radiotherapy. Int J Radiat Oncol Biol Phys 79 : 508-513, 2011   25. Vranic A, Popovic M, Cör A, Prestor B, Pizem J : Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery 67 : 1124-1132, 2010   26. Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA : Atypical meningioma: a study of prognostic factors. World Neurosurg 80 : 549-553, 2013  
|
|



















