Jin, Kim, Yang, Kim, Min, Jeon, Choi, Moon, and Jeong: The Effect of Genetically Modified Lactobacillus plantarum Carrying Bone Morphogenetic Protein 2 Gene on an Ovariectomized Rat
Abstract
Objective
Osteoporosis result from age-related decline in the number of osteoblast progenitors in the bone marrow. Probiotics have beneficial effects on the host, when administered in appropriate amounts. This study investigated the effects of probiotics expressing specific genes, especially the effects of genetically modified bone morphogenetic protein (BMP)-2-expressing Lactobacillus plantarum CJNU 3003 (LP) on ovariectomized rats.
Methods
Twenty-eight female Wistar rats (250-300 g, 12 weeks old) were divided into four groups : the sham (control), the ovariectomy (OVX)-induced osteoporosis group (OVX), the OVX and LP (OVX/LP), OVX and genetically modified BMP-2-expressing LP (OVX/LP with BMP) groups. The three groups underwent bilateral OVX and two of these groups were administered two different types of LP via oral gavage daily. At 16 weeks post-OVX, blood was collected from the heart and the bilateral tibiae were extracted and were scanned by ex-vivo micro-computed tomography and stained with hematoxylin-and-eosin (H&E) and Masson’s trichrome stain for pathological assessment. The serum levels of osteocalcin (OC), rat C-telopeptide of type I collagen (CTX-I), BMP-2, and receptor activator of nuclear factor-ĸB ligand (RANKL) were measured.
Results
The 3D-micro-computed tomography images showed that the trabecular structure in the OVX/LP with BMP group was maintained compared with OVX and OVX/LP groups. No significant differences were detected in trabecular thickness (Tb.Th) between control and OVX/LP with BMP groups (p>0.05). Furthermore, a tendency toward increased BMD, trabecular bone volume, Tb.Th, and trabecular number and decreased trabecular separation was found in rats in the OVX/LP with BMP groups when compared with the OVX and OVX/LP groups (p>0.05). The H&E and Masson’s trichrome stained sections showed a thicker trabecular bone in the OVX/LP with BMP group compared with the OVX and OVX/LP groups. There was no difference in serum levels of OC, CTX and RANKL control and OVX/LP with BMP groups (p>0.05). In contrast, significant differences were found in OC and CTX-1 levels between the OVX and OVX/LP with BMP groups (p<0.05).
Conclusion
Our results showed that the expression of genetically modified BMP-2 showed inhibition effect for bone loss in a rat model of osteoporosis.
Key Words: Probiotics · Osteoporosis · Models, animal · Gene editing · Bone morphogenetic protein 2.
INTRODUCTION
Osteoporosis is an important health concern. It is associated with abnormal bone metabolism in which bone resorption is greater than bone formation [ 4]. The mechanism of bone metabolism is complex, and multiple factors such as genetics, lifestyle and environment play a role [ 30]. Probiotics are living microorganisms [ 9], sometimes derived from fermented food [ 3], which have beneficial effects on the host when administered in appropriate amounts [ 9, 27]. The antiosteoporotic effects of specific probiotic strains such as Lactobacillus casei, L. plantarum, L. paracasei, and Bifidobacterium longum were reported previously [ 25]. Recent reports suggest that gene modification for the synthesis of antioxidant enzymes or cytokines in probiotics may facilitate the development of beneficial strains that can be used in the treatment of various diseases. Furthermore, the design of efficient systems of gene expression and appropriate protein synthesis in probiotics allow application of these microorganisms in the production and secretion of various cytokines or proteins [ 17]. Therefore, probiotics represent possible candidates for medicinal use [ 5]. Therefore, the object of this study was to develop and evaluate the effects of genetically modified bone morphogenetic protein (BMP)-2-expressing Lactobacillus plantarum CJNU 3003 (LP) on ovariectomized rats. To the best of our knowledge, no such study has been conducted or published until now.
MATERIALS AND METHODS
This experiment was approved by the Institutional Animal Care and Use Committee of Asan Institute for Life Sciences Seoul, Korea (2017-14-228).
Construction of recombinant LP (pLR5cat-PSBMP2)
To construct a secretion system expressing BMP-2 gene in lactic acid bacteria, a promoter and secretion signal sequence of the bifidobacterial α-amylase gene was used [ 21]. The promoter and secretion signal sequences were amplified by polymerase chain reaction (PCR) with primers PSamyF (5’- GGG CAT CGC CGA ATA TAC TCC-3’) and PSamyR (5’- GGC CTG TGC GGT GCT GGC-3’) using a recombinant plasmid pLR5cat(S)_PSAB as the PCR template [ 7]. The PCR product was ligated with a murine BMP-2 gene cDNA, which was PCR-amplified with primers BMP2F_Phos (5’- ATG GTG GCC GGG ACC CGC-3’ with 5’ phosphorylation) and BMP2R (5’- CTA ACG ACA CCC GCA GCC-3’) using a commercial plasmid vector pGEM-mBMP2 (Sino Biological Inc., Wayne, PA, USA) as the PCR template. The ligated product was fully amplified by PCR with primers PSamyF and BMP2R and the recombinant DNA PSBMP2 was cloned into pUC18 Escherichia coli ( E. coli) plasmid vector, which was digested with SmaI restriction enzyme (Beams Biotechnology Co. Ltd., Seongnam, Korea). The recombinant DNA pUC18-PSBMP2 was transformed into E. coli DH5α and the nucleotide sequence of PSBMP2 was analyzed with universal primers of pUC18. The PCR-amplified PSBMP2 was cloned into pLR5cat, an E. coli-lactobacilli shuttle vector, which was digested with SmaI enzyme and transformed into E. coli DH5α [ 21]. The recombinant plasmid pLR5cat-PSBMP2 was transferred to LP via electroporation [ 20]. The recombinant LP (pLR5cat-PSBMP2) was confirmed by PCR amplification of PSBMP2.
Animals
Using computerized random numbers, this study was performed with 28 Wistar rat (Orient Bio, Seongnam, Korea) (250-300 g, aged 12 weeks), which were divided into four groups, seven for each group, which is the minimal number for the statistical analysis : sham (control), ovariectomy (OVX)-induced osteoporosis group (OVX), OVX and LP feeding group (OVX/LP), and OVX and genetically modified BMP-2-expressing LP feeding group (OVX/LP with BMP). Three groups underwent bilateral OVX and two of these groups were administered two different types of LP via oral gavage daily.
Ovariectomy
The animals were sedated with 5% isoflurane. Ovariectomy was performed via bilateral dorsal skin incision (4 cm) in the middle. The peritoneal cavity was opened to reveal the ovary surrounded by fat. After blood vessel ligation, the site between the Fallopian tube and the uterine horn was cut and the ovary was removed. The incision required suturing. An intramuscular injection of antibiotic (cefazolin 10 mg/kg, ketoprofen 5 mg/kg) was administered for three postoperative days. Body weight was monitored once weekly for 20 weeks. All animal was treated in accordance with the Guide for the Care and Use of Laboratory Animals.
Probiotic treatment
Probiotic treatment was started 28 days after OVX surgery. During 16 weeks, rats in the OVX/LP group were treated with a single LP and genetically modified BMP-2 expressing LP was administered to the OVX/LP with BMP group at a volume of 400 µL (approximately 108 CFU).
Ex vivo micro-computed tomography (CT) evaluation
Twenty weeks after OVX, all rat were sedated with 5% isoflurane. Blood was drawn via cardiac puncture and were euthanized. Bilateral tibiae were removed and analyzed by high-resolution micro-CT using a Skyscan 1172 micro-CT apparatus (Skyscan NV, Kontich, Belgium). The volume of interest (VOI) was started at a distance of 2.5 mm distally from the growth plate of the proximal tibia and extended towards the diaphysis for 100 slices. In each transverse slice of the VOI, the region of interest was defined as the entire trabecular compartment. The acquisition settings entailed an X-ray source voltage of 49 kV and a current of 200 µA. The pixel size was 19.97 mm. The exposure time was 670 ms and a rotation of 0.4° with complete rotation was achieved. Image reconstruction and analysis were performed using Skyscan software (Skyscan NV). The parameters evaluated included trabecular bone volume (BV/TV; %), trabecular number (Tb.N; /mm), trabecular separation (Tb.Sp; mm), trabecular thickness (Tb.Th; mm), and bone mineral density (BMD; g/cm2).
Hematoxylin and Eosin (H&E) staining
After decalcification, the samples were fixed in paraffin and cut longitudinally at 3 µm thickness using a microtome (mode : RM2255; Leica Microsystems, Wetzlar, Germany). Each sample was mounted on a slide and exposed to a temperature of 55°C. And then, the samples were deparaffinized in four 5-minute changes of xylene and dehydrated in 2-minute changes in decreasing alcohol solutions (100%, 95%, 80%, and 70%) sequentially. The slides were washed in flowing purified water for 1 minute and engrossed in Harris Hematoxylin-I (YD-Diagnostics, Yongin, Korea) for 5 minutes, followed by a 1-minute wash under flowing tap water. Sections were immersed in ammonia water for 30 seconds and then washed under tap water for 3 minutes. The sections were stained with Eosin Y (Sigma-Aldrich, St. Louis, MO, USA) for 2 minutes 30 seconds and dried out from 95% to 100% ethanol. Any residual stain washed away with distilled water. Each section was mounted (Shandon Synthetic Mountant; Thermo Fisher Scientific, Waltham, MA, USA).
Masson’s trichrome staining
After deparaffinization and hydration, slices were treated with a mordant comprising 5% iron alum solution at 56°C dry oven for 30 minutes. Sections were washed in running tap water to remove picric acid for 5 to 6 minutes. Sections were stained with Weigert’s iron hematoxylin solution for 10 minutes, and rinsed with purified water. They were then stained with Biebrich scarlet-acid fuchsin solution (Sigma-Aldrich) for 5 minutes and washed with purified water. The sections were placed in phosphomolybdic-phosphotungstic acid solution for 5 minutes. The solution was discarded and the sections were washed with distilled water. Samples were differentiated in 1% glacial acetic acid solution for 3 minutes. After discarding the solution, the residual stain was washed off with distilled water.
Serum measurements : blood biochemistry, euthanasia, and sampling
Blood was drawn via cardiac puncture and then the animals were euthanized. The blood samples were centrifuged at 3000 rpm for 10 minutes at 4° to separate serum. The serum was removed and stored at -80°C until analysis. Subsequently, the serum levels of osteocalcin (OC), rat C-telopeptide of type I collagen (CTX-I), BMP-2, and receptor activator of nuclear factor-ĸB ligand (RANKL) were measured. OC was determined with a Rat-Mid OC ELISA kit (IDS, Fareham, UK), CTX-I with a Rat CTX-I ELISA Kit (Cusabio, Houston, TX, USA), BMP-2 with a Quantikine BMP-2 Immunoassay (Cusabio), and RANKL using a Rat RANK ELISA kit (Cusabio). The serum parameters were based on their absorbance using a microplate absorbance reader (model Sunrise; TECAN, Seestrasse, Männedorf, Switzerland) according to the manufacturer’s instruction.
Statistical analysis
Data are expressed as means±standard error of mean or standard deviations. Two-way analysis of variance (ANOVA) with Bonferroni post-test correction for body weight and Mann-Whitney U test for micro-CT measurements were conducted to determine whether the parameters differed significantly between groups. A p-value of 0.05 or less was considered statistically significant. All analyses were performed using R (version 3.3.2; The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Construction of recombinant LP (pLR5cat-PSBMP2)
PSBMP2, in which a promoter and secretion signal sequence of the bifidobacterial α-amylase gene was linked to a murine BMP-2 gene cDNA, was successfully amplified by PCR and cloned into the pUC18 plasmid vector. The nucleotide sequence of the PSBMP2 was analyzed using the universal primers of pUC18 vector to confirm that the promoter and secretion signal sequence was correctly ligated with the BMP-2 gene cDNA (data not shown). The PCR-amplified PSBMP2 was cloned into pLR5cat, an E. coli-lactobacilli shuttle vector, and transferred to LP. We verified the recombinant LP (pLR5cat-PSBMP2) by PCR where PSBMP2 of the correct size was amplified ( Fig. 1).
Body weight
All animals tolerated the procedure well. There was no wound dehiscence or local infection. Furthermore, there were no complications associated with implanted devices during the study period. The mean body weight of rats in all groups increased over a period of 20 weeks after ovariectomy. Weights of the rats in the OVX, OVX/LP, OVX/LP with BMP groups were significantly higher than in the control group ( p<0.05). The body weight of rats in the OVX/LP and OVX/LP with BMP groups was slightly lower than in the OVX group ( p>0.05; Fig. 2).
Micro-CT measurements
Using 3D-micro-CT, osteoporotic changes of bone were observed in rats in the OVX, OVX/LP, and OVX/LP with BMP groups; however, the trabecular structure in the OVX/LP with BMP group was maintained compared with OVX and OVX/LP groups ( Fig. 3). The control group had the highest values of BMD, BV/TV, Tb.Th and Tb.N and the lowest values of Tb.Sp compared with the OVX, OVX/LP, OVX/LP with BMP groups significantly ( p<0.05; Fig. 4). However, no significant differences were detected in Tb.Th between control and OVX/LP with BMP groups ( p>0.05). Furthermore, a tendency toward increased BMD, BV/TV, Tb.Th, and Tb.N and decreased Tb.Sp was found in rats in the OVX/LP with BMP groups when compared with the OVX and OVX/LP groups, which was not significant statistically ( p>0.05; Fig. 4).
Histology
During the 20 weeks post-ovariectomy, H&E and Masson’s trichrome-stained sections of the tibia showed a decrease of bony trabeculae and increased adipose tissue infiltration in the bone marrow of rats in OVX, OVX/LP and the OVX/LP with BMP groups when compared with those of the control group. However, the trabecular formation in the OVX/LP with BMP group was retained compared with the OVX and OVX/LP groups ( Fig. 5).
Changes in the serum levels of biochemical markers
The serum level of BMP-2 was higher in the control group compared with that of the other three groups, with the lowest level observed in the OVX group ( p<0.05). Furthermore, the serum levels of the BMP in the OVX/LP, and OVX/LP with BMP groups were higher than that of OVX. Unfortunately, there was no significant difference in serum level of BMP-2 between the OVX, OVX/LP, and OVX/LP with BMP groups ( p>0.05). Serum levels of OC, CTX, and RANKL were the highest in the OVX group and there was a significant difference between OVX and control group ( p<0.05). However, there was no difference in serum levels of OC, CTX, and RANKL between control and OVX/LP with BMP group ( p>0.05). In contrast, significant differences were found in OC and CTX-I levels between the OVX and OVX/LP with BMP group ( p<0.05) ( Fig. 6).
DISCUSSION
Multiple animal models were designed to study postmenopausal osteoporosis [ 13, 36]. Because of the low price and easy manipulation, the OVX rat model represents a standard tool for the investigation of postmenopausal osteoporosis. This model typically shows cancellous bone defects such as postmenopausal bone loss [ 13, 36] and is also used as an obesity model. At the end of this study, the mean body weight increased in all groups during the 20 weeks post-ovariectomy and all the OVX groups showed similar body weight, which was significantly higher than in the control group, indicating to forming a good experimental model for the postmenopausal osteoporosis study ( Fig. 2). It is theoretically possible that treatment with genetically modified probiotics is associated with health benefits. Due to the availability of genomic data, genetic manipulation is easy and various genetic tools have been developed. A few Lactobacillus species are used in food manufacturing procedures [ 5]. Using molecular techniques, vectors have been employed in gene cloning, and their products can be managed with promoters [ 5]. Therefore, we constructed the recombinant LP (pLR5cat-PSBMP2), which was confirmed by PCR via amplification of PSBMP2. Osteoporosis is due to an age-linked decline in the number of osteoblast progenitors in the bone marrow as well as decreasing estrogen levels [ 8]. The prevalence of osteoporosis is 10.3% and the prevalence of low bone mass is 43.9% in adults aged 50 years and older in the USA [ 39]. Many drugs for osteoporosis have been developed and antiresorptive drugs, such as bisphosphonates and selective estrogen receptor modulators are the mainstay of treatment for osteoporosis [ 24]. Recent studies reported the role of anabolic drugs such as Teriparatide (Forsteo ® from Lilly). However, anabolic drugs, which activate osteoblastic stem cells to increase bone formation and enhance bone strength still require for the appropriate treatment [ 15]. In spite of beneficial effects of probiotics in various diseases [ 6, 12, 38], only a few studies investigated the effects of probiotics on osteoporosis [ 26]. In a previous study, specific probiotic strains showed potential for the preservation of bone structure and prevention of osteoporosis [ 18, 32, 33]; exposure to Lactococcus spp. reduced age-related bone loss in senescence-accelerated mice [ 16] and Lactobacillus reuteri ATCC PTA 6475 improved bone density in mice [ 19], and Bacillus licheniformis and Bacillus subtilis increased cortical bone mineral content in chicken [ 23]. In our study, we used genetically modified BMP-2-expressing LP and evaluated the BMD and other parameters via micro-CT 20 weeks post-OVX. A trend towards an increase in BMD, BV/TV, Tb.Th, and Tb.N and a decrease in Tb.Sp of the OVX/LP with BMP group was observed when compared with the OVX and OVX/LP groups, although the differences were not statistically significant. Furthermore, no significant differences existed in Tb.Th levels between control and OVX/LP with BMP group ( Fig. 4) and the trabecular formation was more preserved in the OVX/LP with BMP group compared with the OVX and OVX/LP group in pathologic findings ( Fig. 5). Therefore, genetically modified BMP-2-expressing LP prevented osteoporotic changes in OVX rat. BMP is a sub-family of transforming growth factor-β first identified by Urist [ 37] and to be osteoinductive and osteogenic for mesodermal cells [ 2, 40]. BMP plays a role in skeletal development, especially in early embryogenesis [ 34] and promotes cell growth and differentiation [ 2, 11]. Unfortunately, systemic effect of the BMP-2 is not revealed exactly in adult human. However, previous study exhibited osteoporotic fracture treatment with BMPs induced rapid increase of bone strength, especially at a local area of bone fracture [ 14]. In this study, the serum level of BMP-2 was higher in the control group when compared with the other three groups, with the lowest level observed in the OVX group ( p<0.05). However, the BMP-2 concentration in rats belonging to the OVX/LP and OVX/LP with BMP groups were higher than in the OVX group ( p>0.05; Fig. 6). Therefore, we assumed that the rise in serum BMP-2 expression resulted in an increase in BMP-2 secretion of the gene modified LP and absorption by cells of the intestinal mucosa. These factors might have prevented the osteoporotic changes in rats of the OVX with BMP group. Bone undergoes remodeling by replacing old and damaged tissue with new bone. Osteoclasts and osteoblasts work consecutively in the same bone remodeling unit. Bone remodeling is most prominent on the cancellous bone surfaces, where osteoclasts remove the eroded surface in the old and damaged bone [ 1]. Sequentially, bone formation is induced by osteoblasts. During the bone resorption stage, serum CTX is released by osteoclasts. Therefore, CTX is a bone resorption marker and reflects osteoclast activity. Furthermore, RANKL is a member of the tumor necrosis factor family and also binds to RANK on osteoclasts, as an important mediator of osteoclast differentiation and activation. Thus, the increase in bone resorption occurs together with increased osteoclast activity and increased levels of CTX and RANKL in the serum. In the present study, we determined serum CTX and RANKL levels, and the levels of CTX and RANKL in the OVX group were the highest and statistically significant compared with the control group. However, levels in the OVX/LP with BMP group were not significantly different from those in control. Moreover, there was significant improvement between the OVX group and the OVX/LP with BMP group in CTX level ( p<0.05; Fig. 6). Unfortunately, there was no difference in serum levels of RANKL between OVX and OVX/LP with BMP group ( p>0.05; Fig. 6). Nonetheless, we thought that the genetically modified BMP-2-expressing LP treatment can improve the osteoporosis via prevent the activation of the osteoclasts. Serum OC is one of the bone formation marker secreted by osteoblasts and osteocytes [ 35]. During the bone formation process, increases in osteoblast activity and OC levels in the serum also occur [ 10, 29]. Therefore, the activity of osteoblasts can be determined by monitoring OC level [ 28]. In this study, the serum levels of OC were significantly increase of the OVX group than control group ( p<0.05; Fig. 6). This finding suggests increase of osteoblast activation by osteoclast activity increase in OVX osteoporotic rats as physiologic mechanism of coupling bone resorption and formation. However, the serum level of OC in the OVX/LP with BMP group was not significantly different compared with the control group ( p>0.05). This finding is indicating the beneficial effect of genetically modified BMP-2-expressing LP treatment in preventing osteoporotic changes This study had limitations that could be debated. First, this research only measured the amplification of PSBMP2 from the recombinant probiotics by PCR and did not evaluate Western blots to confirm the expression of the corresponding protein [ 31]. Second, we didn’t perform immunohistochemistry in the tibia for evaluating BMP-2, alkaline phosphatase, osteocalcin, osteopontin, runt-related transcription factor 2, CTX1, RANKL in bone tissue and we also did not perform western blots for the proteins from tibia. Third, short-chain fatty acids can reduce secondary acid formation in the colon, decrease luminal pH, and increase mineral absorption subsequently [ 22, 26]. Fourth, we only evaluated BMD via ex-vivo micro-CT at the time of OVX and at 24 weeks. Therefore, we could not get serial data in the same subjects. Consecutive in vivo micro-CT scans would have been suitable for checking the rate of BMD reduction.
CONCLUSION
Our results showed that the expression of genetically modified BMP-2 showed inhibition effect for bone loss in a rat model of osteoporosis.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant no. NRF-2017R1A2B1005327), and this work was supported by the Soonchunhyang University Research Fund.
This document has been checked by at least two professional editors, both of whomare native English speakers.
Fig. 1.
Confirmation of recombinant Lactobacillus plantarum CJNU 3003 (LP; pLR5cat-PSBMP2) by polymerase chain reaction (PCR). Escherichia coli DH5α (pLR5cat-PSBMP2) as positive control for PCR template (a); LP (pLR5cat-PSBMP2) candidate (b). Lane 1, 4 : PSamyF and PSamyR primer set, Lane 2, 5 : bone morphogenetic protein (BMP)-2 factor and BMP-2 receptors primer set, Lane 3, 6 : PSamyF-BMP2R primer set, Lane M : size marker (MG 1 kb plus DNa ladder marker; MGmed Co., Seoul, Korea). 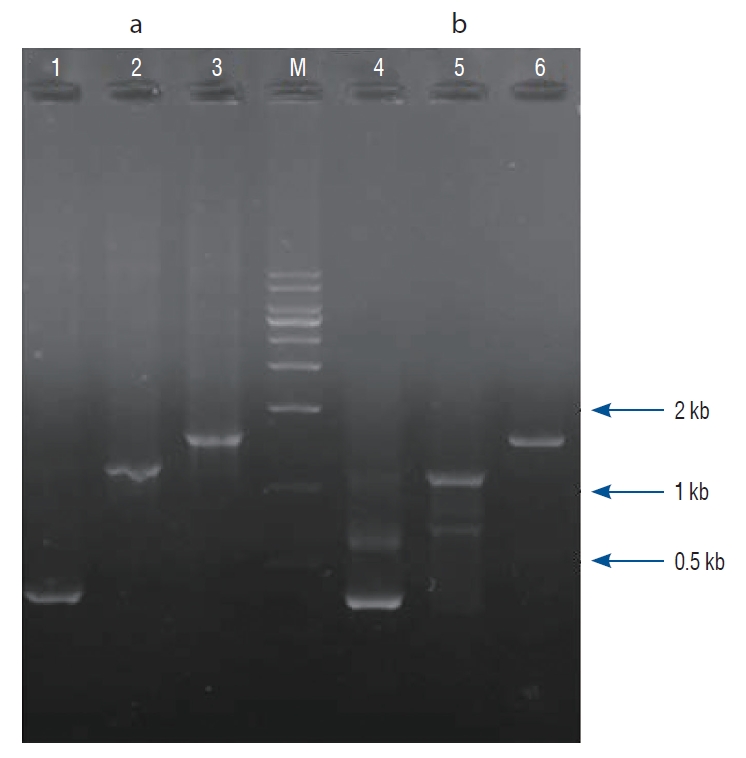
Fig. 2.
Graphic analysis of body weight. In all groups, the mean body weight increased over 20 weeks after ovariectomy-induced osteoporosis group (OVX). Weights of rats in the OVX, OVX and Lactobacilus plantarium CJNU 3003 (LP) feeding group (OVX/LP), OVX and genetically modified bone morphogenetic protein (BMP)-2-expressing LP feeding group (OVX/LP with BMP) were higher than in the control group (sham group). Significantly, the body weight in the OVX/LP, OVX/LP with BMP group was slightly lower than that in the OVX group. All data are expressed as the mean±standard error of mean. Two-way aNOVa, bonferroni post-tests *p<0.05, †p<0.01, ‡p<0.001 vs. control. 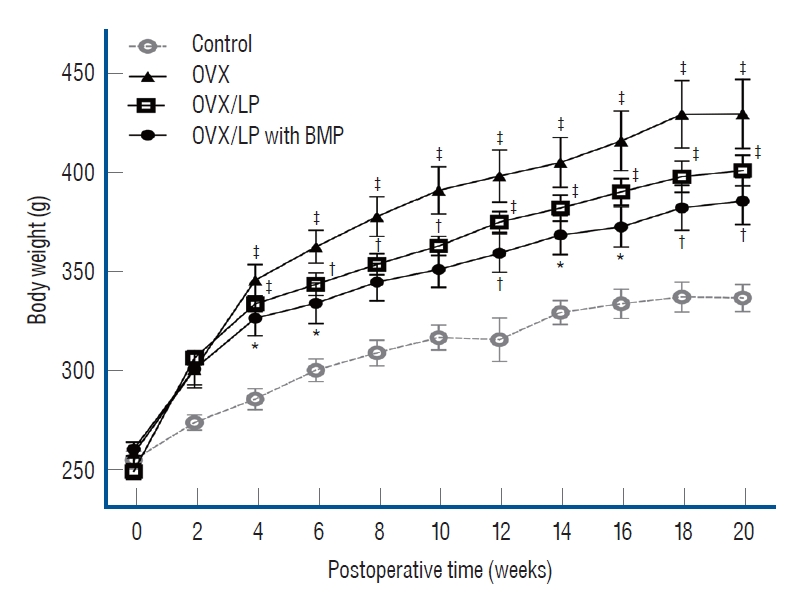
Fig. 3.
A representative micro-computed tomography image. In spite of osteoporotic changes in rats in the ovariectomy-induced osteoporosis group (OVX), OVX and Lactobacilus plantarium CJNU 3003 (LP) feeding group (OVX/LP), OVX and genetically modified bone morphogenetic protein (BMP)-2-expressing LP feeding group (OVX/LP with BMP), the trabecular structure was highly preserved in the OVX/LP with BMP group than in OVX and OVX/LP groups. Control, sham group. 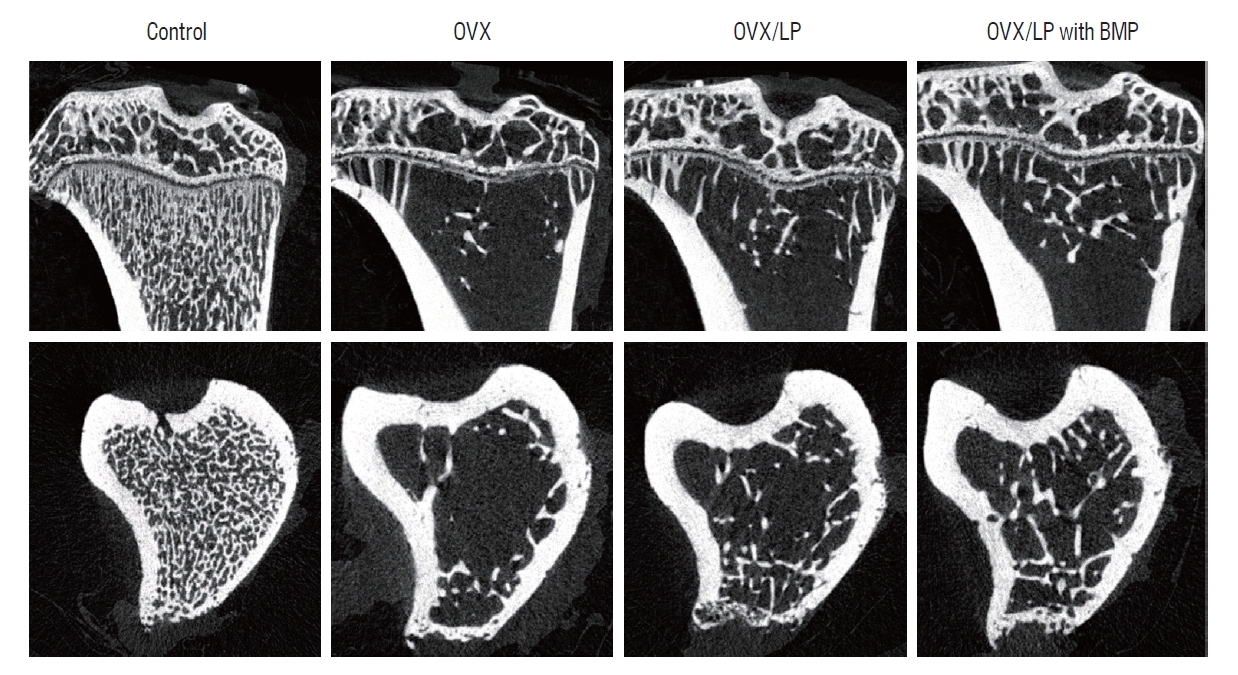
Fig. 4.
Graphic analysis of micro-computed tomography evaluation. The control group (sham group) had the highest values of bone mineral density (BMD), trabecular bone volume (BV/TV), trabecular thickness (Tb. Th), and trabecular number (Tb.N) and the lowest values of trabecular spacing (Tb.Sp). The differences in Tb.Th between control and ovariectomy (OVX) and genetically modified bone morphogenetic protein (BMP)-2-expressing Lactobacilus plantarium CJNU 3003 (LP) feeding group (OVX/LP with BMP) were not significant. All data are expressed as the mean±standard deviation. *p<0.05, †p<0.01, ‡p<0.001 vs. control. 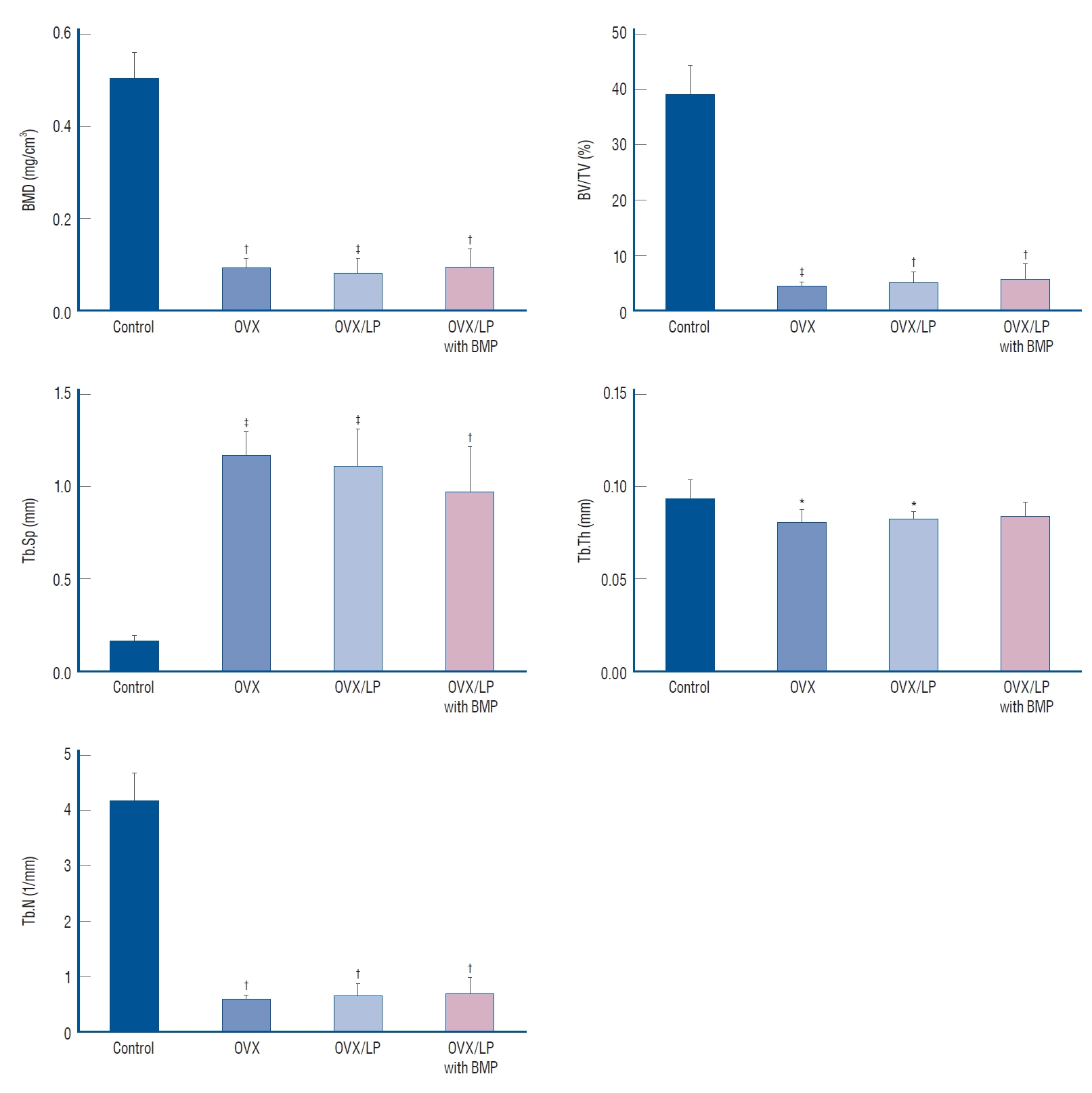
Fig. 5.
Images of (A) hematoxylin and eosin (H&E) and (B) Masson’s trichrome staining of tibia. The images show loss of bony trabeculae and increased adipose tissue in the bone marrow of rats in the ovariectomy-induced osteoporosis group (OVX), OVX and Lactobacilus plantarium CJNU 3003 (LP) feeding group (OVX/LP), OVX and genetically modified bone morphogenetic protein (BMP)-2-expressing LP feeding group (OVX/LP with BMP). However, there are more preservation of the trabecular formation in the OVX/LP with BMP group compared to the OVX and OVX/LP group. Control, sham group. 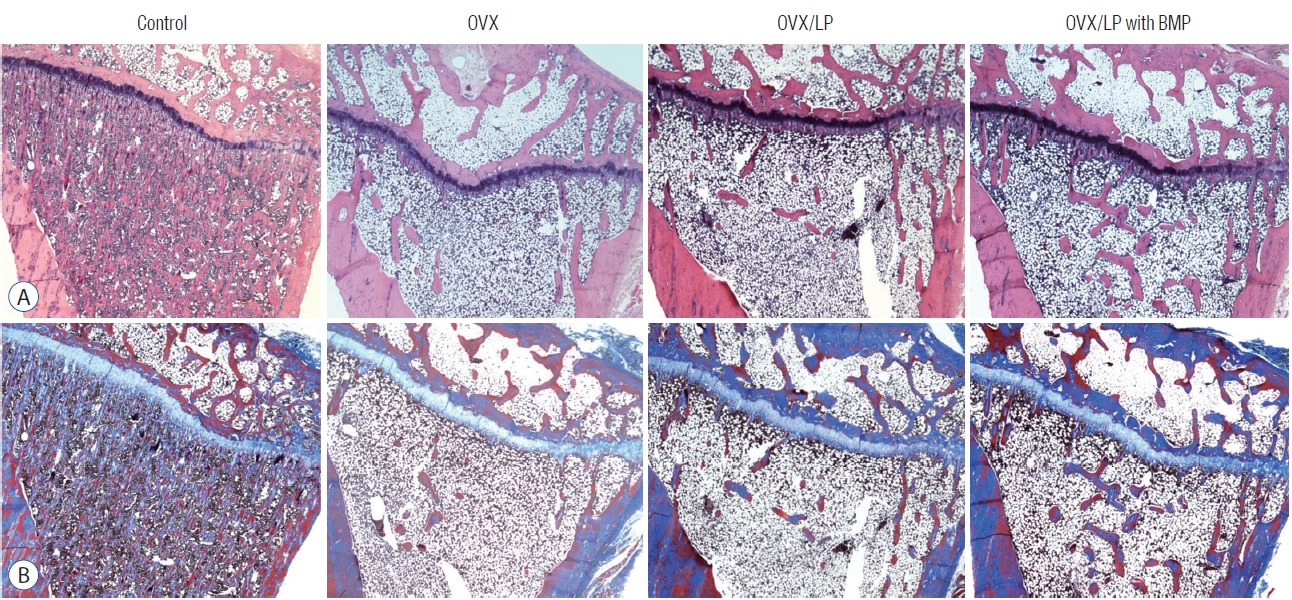
Fig. 6.
Changes in the levels of serum markers. A : The serum level of bone morphogenetic protein (BMP)-2 was higher in the control group (sham group) than in the other three groups, with the lowest level observed in the ovariectomy-induced osteoporosis group (OVX) group (p<0.05). There is no significant difference in serum biomarker levels between the OVX, OVX and Lactobacilus plantarium CJNU 3003 (LP) feeding group (OVX/LP), and OVX and genetically modified BMP-2-expressing LP feeding group (OVX/LP with BMP) (p>0.05). B-D : Serum levels of osteocalcin (OC), C-telopeptide of type I collagen (CTX-I), and receptor activator of nuclear factor-ĸB ligand (RaNKL) were the highest in the OVX group, with significant differences between OVX and control group. However, there is no difference between control and OVX/LP with BMP (p>0.05). In contrast, significant differences in OC and CTX-1 existed between the OVX and OVX/LP with BMP groups (p<0.05). All data are expressed as the mean±standard deviation. *p<0.05, †p<0.01, ‡p<0.001 vs. control, §p<0.05, =p<0.01, ¶p<0.001 vs. OVX. 
References
1. Abdul-Majeed S, Mohamed N, Soelaiman IN : Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complement Alternat Med 2012 : 960742, 2012    2. Astrand P, Ahlqvist J, Gunne J, Nilson H : Implant treatment of patients with edentulous jaws: a 20-year follow-up. Clin Implant Dent Relat Res 10 : 207-217, 2008   3. Chilton SN, Burton JP, Reid G : Inclusion of fermented foods in food guides around the world. Nutrients 7 : 390-404, 2015    4. Christiansen C : Prevention and treatment of osteoporosis: a review of current modalities. Bone 13 Suppl 1 : S35-S39, 1992  5. de Moreno de LeBlanc A, Del Carmen S, Chatel JM, Miyoshi A, Azevedo V, Langella P, et al : Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract 2015 : 146972, 2015    6. de Vrese M, Schrezenmeir J : Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 111 : 1-66, 2008   7. Eom JE, Moon SK, Moon GS : Heterologous production of pediocin PA-1 in Lactobacillus reuteri. J Microbiol Biotechnol 20 : 1215-1218, 2010   8. Friedenstein AJ : Precursor cells of mechanocytes. Int Rev Cytol 47 : 327-359, 1976   9. Howarth GS, Wang H : Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients 5 : 58-81, 2013    10. Ivaska KK, Hentunen TA, Vääräniemi J, Ylipahkala H, Pettersson K, Väänänen HK : Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem 279 : 18361-18369, 2004   11. Jung UW, Choi SY, Pang EK, Kim CS, Choi SH, Cho KS : The effect of varying the particle size of beta tricalcium phosphate carrier of recombinant human bone morphogenetic protein-4 on bone formation in rat calvarial defects. J Periodontol 77 : 765-772, 2006   12. Kailasapathy K, Chin J : Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 78 : 80-88, 2000   13. Kalu DN : The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15 : 175-191, 1991   14. Kanakaris NK, Petsatodis G, Tagil M, Giannoudis PV : Is there a role for bone morphogenetic proteins in osteoporotic fractures? Injury 40 Suppl 3 : S21-S26, 2009   15. Khosla S, Westendorf JJ, Oursler MJ : Building bone to reverse osteoporosis and repair fractures. J Clin Invest 118 : 421-428, 2008    16. Kimoto-Nira H, Suzuki C, Kobayashi M, Sasaki K, Kurisaki J, Mizumachi K : Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr 98 : 1178-1186, 2007   17. LeBlanc JG, Aubry C, Cortes-Perez NG, de Moreno de LeBlanc A, Vergnolle N, Langella P, et al : Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: an update. FEMS Microbiol Lett 344 : 1-9, 2013   18. Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, et al : Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res 15 : 13-19, 2000   19. McCabe LR, Irwin R, Schaefer L, Britton RA : Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 228 : 1793-1798, 2013    20. Moon GS, Lee YD, Kim WJ : Screening of a novel lactobacilli replicon from plasmids of Lactobacillus reuteri KCTC 3678. Food Sci Biotechnol 17 : 438-441, 2008
21. Moon GS, Pyun YR, Park MS, Ji GE, Kim WJ : Secretion of recombinant pediocin PA-1 by Bifidobacterium longum, using the signal sequence for bifidobacterial alpha-amylase. Appl Environ Microbiol 71 : 5630-5632, 2005    22. Moslehi-Jenabian S, Pedersen LL, Jespersen L : Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2 : 449-473, 2010    23. Mutuş R, Kocabagli N, Alp M, Acar N, Eren M, Gezen SS : The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci 85 : 1621-1625, 2006   24. Park SB, Lee YJ, Chung CK : Bone mineral density changes after ovariectomy in rats as an osteopenic model : stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc 48 : 309-312, 2010    26. Parvaneh K, Jamaluddin R, Karimi G, Erfani R : Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal 2014 : 595962, 2014    27. Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, et al : New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol 37 : 105-118, 2003   28. Romero Barco CM, Manrique Arija S, Rodríguez Pérez M : Biochemical markers in osteoporosis: usefulness in clinical practice. Reumatol Clin 8 : 149-152, 2012   29. Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, et al : Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int 66 : 100-103, 2000   30. Rubin CJ, Lindberg J, Fitzsimmons C, Savolainen P, Jensen P, Lundeberg J, et al : Differential gene expression in femoral bone from red junglefowl and domestic chicken, differing for bone phenotypic traits. BMC Genomics 8 : 208, 2007    31. Sakata T, Kojima T, Fujieda M, Miyakozawa M, Takahashi M, Ushida K : Probiotic preparations dose-dependently increase net production rates of organic acids and decrease that of ammonia by pig cecal bacteria in batch culture. Dig Dis Sci 44 : 1485-1493, 1999  32. Scholz-Ahrens KE, Schrezenmeir J : Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr 137( 11 Suppl):2513S-2523S, 2007   33. Shim KS, Kim T, Ha H, Lee KJ, Cho CW, Kim HS, et al : Lactobacillus fermentation enhances the inhibitory effect of Hwangryun-haedok-tang in an ovariectomy-induced bone loss. BMC Complement Altern Med 13 : 106, 2013    34. Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, et al : Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol 1 : E69, 2003    35. Szulc P, Delmas PD : Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int 19 : 1683-1704, 2008   36. Turner AS : Animal models of osteoporosis--necessity and limitations. Eur Cell Mater 1 : 66-81, 2001   37. Urist MR : Bone morphogenetic protein: the molecularization of skeletal system development. J Bone Miner Res 12 : 343-346, 1997   38. Vitetta L, Briskey D, Alford H, Hall S, Coulson S : Probiotics, prebiotics and the gastrointestinal tract in health and disease. Inflammopharmacology 22 : 135-154, 2014   39. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al : The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29 : 2520-2526, 2014   40. Yamaguchi K, Masuhara K, Yamasaki S, Nakai T, Fuji T : Cyclic therapy with etidronate has a therapeutic effect against local osteoporosis after cementless total hip arthroplasty. Bone 33 : 144-149, 2003  
|
|






















