INTRODUCTION
Primary intracranial undifferentiated sarcoma is an extremely rare entity. Undifferentiated sarcomas, formerly known as malignant fibrous histiocytomas (MFH), are usually identified in the soft tissue of the extremities and trunk2,1013). A literature review of intracranial undifferentiated sarcomas, including the formerly used less-specific term MFH, identified less than three dozen cases reports since 1976 6,912,16). Among these case reports of intracranial sarcomas, very few describe a transformation from pre-existing intracranial tumors. Intracranial sarcomas developed after radiosurgery in three cases 11,1214) and after chemotherapy in one case1). These reports suggest that intracranial undifferentiated sarcomas can be derived by the transformation of other tumors of the nervous system. However, the exact mechanism and risk factors remain undefined.
Here, we report our experience in managing a rapidly growing intracranial undifferentiated sarcoma in a young adult who was initially diagnosed with a low-grade glioma. To the best of our knowledge, this is the first report of undifferentiated sarcoma arising from low-grade glioma without any chemotherapy or radiotherapy.
CASE REPORT
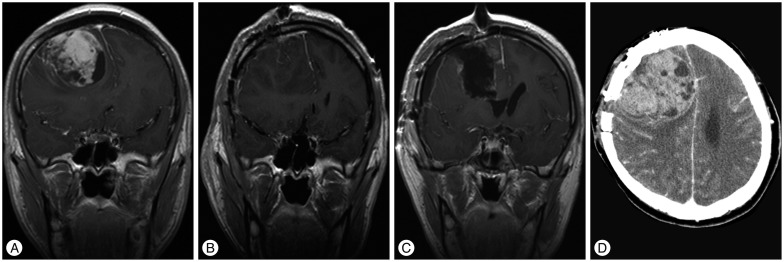
A 23-year-old man presented with headache and loss of consciousness. Brain magnetic resonance (MR) imaging revealed an intra-axial, non-enhancing solitary lesion in the right frontal lobe, which was hyperintense on fluid-attenuated inversion recovery (FLAIR) imaging (Fig. 1A, B). MR spectroscopy (MRS) demonstrated a prominent choline (Cho) peak and a reduction in N-acetyl-aspartate (NAA) (Fig. 1C, D). A stereotactic biopsy specimen was obtained from the area that showed the highest Cho/NAA ratio (approximate value of 2.0). Histopathological tests identified a diffuse astrocytoma [World Health Organization (WHO) grade II]. As per the parent's wishes of postponing any further tumor removal, the patient was discharged at that point of time. Three months later, the patient presented with severe headache and was admitted. Brain MR imaging showed a right frontal mass with strong enhancement and central necrosis (Fig. 2A). The patient was scheduled to undergo surgical removal of the tumor. A hypervascular, friable, intraparenchymal mass was identified, and gross total removal was achieved (Fig. 2B). The final histopathological diagnosis was undifferentiated sarcoma. Postoperatively, the patient showed no definite neurologic deficit. One month later, he was discharged and was followed-up with outpatient adjuvant radiotherapy. However, five months after tumor removal, he visited the emergency room with severe headache. Brain MR imaging showed tumor recurrence. A second tumor removal operation was performed (Fig. 2C). Postoperatively, the patient suffered left hemiparesis caused by radical tumor resection. Two months later, he died of severe brain herniation caused by extensive regrowth and bleeding of the intracranial sarcoma (Fig. 2D).
Histopathological findings
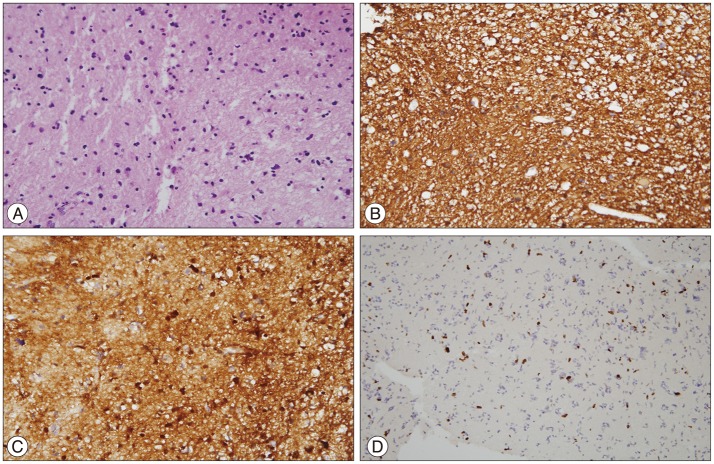
Tumor tissue was obtained by stereotactic biopsy (Fig. 3). Although the tumor tissue showed atypism and higher cellularity than normal brain tissue, there was no mitosis, necrosis, or vascular proliferation (Fig. 3A). On immunohistochemistry, staining for glial fibrillary acidic protein (GFAP) (Fig. 3B) and S100 protein (Fig. 3C) was positive and the Ki-67 labeling index was 3% (Fig. 3D). These findings were compatible with diffuse astrocytoma, WHO grade II.
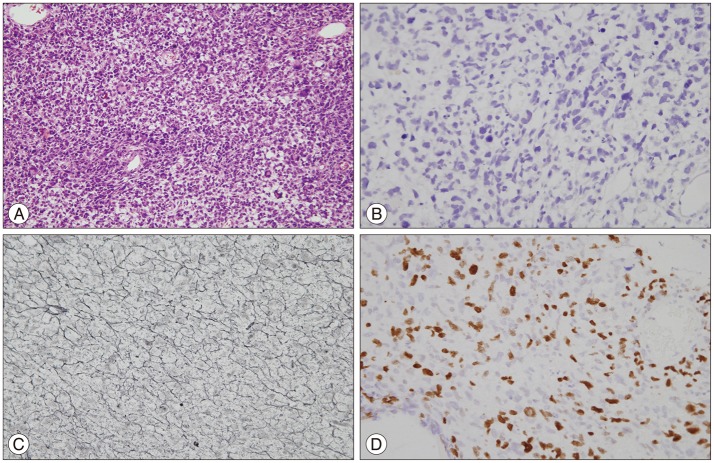
Tissue obtained from the surgical removal of the tumor (Fig. 4) showed significantly increased cellularity and pleomorphic hyperchromatic nuclei. Mitoses were frequently observed. Giant cells and spindle-shaped cells were also seen (Fig. 4A). On immunohistochemistry, staining for GFAP and S100 protein were negative (Fig. 4B). Reticulin staining revealed cells encapsulated by reticulin fibers (Fig. 4C), which is a typical finding of sarcomas. The Ki-67 labeling index was as high as 20% (Fig. 4D). There were no features characteristic of glial tumors.
DISCUSSION
According to the latest WHO classification, the tumor case presented here can be classified as an undifferentiated sarcoma3). Undifferentiated sarcomas can present as round cells, spindle cells, epithelioid cells, or pleomorphic cells and do not resemble any mature tissue4). Because undifferentiated sarcoma is a novel entity, a literature search of previous reports was performed by using the formerly used term "MFH".
Radiologically, intracranial undifferentiated sarcomas resemble high-grade gliomas, which may present with heterogeneous contrast enhancement, central necrosis, and surrounding edema15). In our patient, perilesional edema was not severe, but the heterogeneous rim enhancing pattern and previous histopathological report suggested a high-grade glioma, and follow-up computed tomography imaging demonstrated recurrent tumor bleeding. We concluded that the MR imaging findings of the undifferentiated sarcoma reflected the aggressive nature of the tumor.
The tumor was locally progressive without any evidence of distant metastasis. Despite the repeated tumor resections, recurrence and bleeding caused by the rapid growth of the tumor resulted in the fatal outcome. Although the histopathological findings are similar, we opine that intracranial undifferentiated sarcomas are a different entity from extracranial sarcomas. Based on the previous reports, the mean age of the intracranial sarcoma cases was 38.7 years9) while our patient was 23 years old; the mean age in the extracranial cases was reported to be approximately 60 years2,7). Since extirpation of intracranial tumors is more difficult, the survival period of the intracranial undifferentiated sarcoma is much shorter. In the review of 32 intracranial MFHs by Mitsuhashi et al.9), most of the patients with intracranial MFH died within 1 year from diagnosis. In contrast, the overall 5-year survival of primary undifferentiated sarcomas arising from the extremities has been reported to be over 70%2,7).
One of the possible explanations of how this uncommon sarcoma arises from low-grade glioma is pre-existing heterogeneity of two pathologies since the initial tumor diagnosis. Minimal invasive biopsy surgery is limited in its scope, and there is a possibility of sampling error. The rapidly growing undifferentiated tumor might supersede the comparatively slow growth of low-grade glioma in later stages. Another possibility is that the malignant transformation was triggered by surgical trauma. The initial MR images and tumor tissue obtained by stereotactic biopsy did not show any findings suggesting a high-grade tumor. However, after the biopsy, the tumor underwent rapid progression. Although there are no references on this kind of mechanism in the literature, few investigators have speculated the potential role of wounds in oncogenesis. Hofer et al.5), in an animal study, reported the increased tumor growth after surgical wounding. Meng et al.8), described that wounds can be a risk factor for cancer by means of oncogene activation in wound healing process.
According to the literature on undifferentiated sarcomas of extremities, radical resection of the tumor is the treatment of choice and chemotherapy and radiotherapy are also helpful in lowering the recurrence rate2,10). However, in cases of intracranial undifferentiated sarcomas, authors have reported recurrence and poor prognosis after recommended treatment. Chemoradiotherapy shows temporary improvement but does not change the prognosis dramatically6,911). In our case, the patient received adjuvant radiotherapy after the first resection of the sarcoma. However, the patient showed tumor recurrence after five months.
It is known that subtotally resected low-grade gliomas can transform to high-grade tumors, and in cases of undifferentiated sarcoma, the course is rapid and fatal. Rapidly growing refractory intracranial tumors are challenging for neurosurgeons. Although it is not usual, sporadic reports about malignant transformation by radiation, chemotherapy, and surgical trauma suggest that the early tumor resection is more ideal in young adults.
CONCLUSION
We report our experience in managing a fatal case of intracranial undifferentiated sarcoma. The tumor was locally progressive without any evidence of distant metastasis. However, the treatment strategy of intracranial undifferentiated sarcoma is not established yet. This is the first report of undifferentiated sarcoma arising from low-grade glioma without radiation or any other antitumor therapy. We hope this report will provide information for a better understanding of the pathophysiology of undifferentiated sarcomas.