Jin, Min, Jeon, Choi, and Jeong: Analysis of Molecular Expression in Adipose Tissue-Derived Mesenchymal Stem Cells : Prospects for Use in the Treatment of Intervertebral Disc Degeneration
Abstract
Objective
Recent studies have shown encouraging progress toward the use of autogenic and allogenic mesenchymal stem cells (MSCs) to arrest, or even lead to partial regeneration in, intervertebral disc (IVD) degeneration. However, this technology is still in its infancy, and further development is required. The aim of this study was to analyze whether rat adipose-derived mesenchymal stem cells (ADMSC) can differentiate towards IVD-like cells after treatment with transforming growth factor ╬▓3 (TGF-╬▓3) in vitro. We also performed quantitative analysis of gene expression for ADMSC only, ADMSCs treated with TGF-╬▓3, and co-cultured ADMSCs treated with TGF-╬▓3.
Methods
ADMSCs were sub-cultured to homogeneity and used in fluorocytometry assays for CD11, CD45, and CD90/Thy1. ADMSCs were differentiated in spheroid culture towards the chondrogenic lineage by the presence of TGF-╬▓3, dexamethasone, and ascorbate. We also co-cultured pure ADMSCs and nucleus pulposus cells in 24-well plates, and performed immunohistochemical staining, western blotting, and RT-PCR for quantitativeanalysis of gene expression.
Results
Results of fluorocytometry were positive for CD90/Thy1 and negative for CD11 and CD45. TGF-╬▓3-mediated induction of ADMSCs led to the expression of the differentiation markers of intervertebral disc-like cells, such as aggrecan, collagen II, and sox-9. Co-cultured ADMSCs treated with TGF-╬▓3 showed higher expression of differentiation markers and greater extracellular matrix production compared with ADMSCs treated with TGF-╬▓3 alone.
Conclusion
ADMSC treated with TGF-╬▓3 may be an attractive source for regeneration therapy in degenerative IVD. These findings may also help elucidate the pathologic mechanism of MSC therapy in the degeneration of IVD in vivo.
Key Words: Adipose tissue ┬Ě Mesenchymal stem cells ┬Ě Intervertebral disc ┬Ě Cell and tissue engineering.
INTRODUCTION
Stem cell-mediated cell transplantation can reconstruct the normal structure and function of degenerated intervertebral discs (IVDs) by supplementing the loss of nucleus pulposus cells and promoting the formation of extracellular matrix 7). Recent studies have shown encouraging progress toward the use of autogenic and allogenic mesenchymal stem cell (MSCs) to arrest, or even lead to partial regeneration of, IVD degeneration in various animal models 9,10). However, it is clear that this technology is still in its infancy, and further developments are required while many issues await clarification 5). One major issue is the identification of a suitable cell population with an expression profile resembling that of native IVD tissue and the capacity to regenerate this tissue. An additional concern is the acquisition of the cells in sufficient quantities for application in a clinical setting 11). Further, a greater understanding of the biologic mechanism of MSC transplantation, which remains uncertain, would be helpful in developing therapies. Therefore, we analyzed whether rat adipose-derived stem cells (ADMSCs) can differentiate towards IVD-like cells following transforming growth factor ╬▓3 (TGF-╬▓3)-mediated induction in vitro. We also performed quantitative analysis of gene expression for ADMSC only, ADMSCs treated with TGF-╬▓3, and co-cultured ADMSCs treated with TGF-╬▓3, and evaluated the biologic mechanism of MSC therapy for regeneration of IVD tissue.
MATERIALS AND METHODS
Adipose-derived mesenchymal stem cell (ADMSC) isolation
Female Sprague-Dawley (SD) rats were used in this study. Approximately 10 g of fat tissue was harvested from the bilateral inguinal area. Harvested fat tissue was washed in saline and digested in 0.075% collagenase (filtered with a 0.20-┬Ám syringe filter) at 37Ôäâ for 1 h. The harvested fat tissue was treated with an equal volume of low-glucose Dulbecco's modified Eagle's medium (DMEM : Gibco, Invitrogen) containing 10% fetal bovine serum (Gibco, Invitrogen) and 1% penicillin/streptomycin (Gibco, Invitrogen). Cells were collected by centrifugation for 10 min at 600├Śg, and the supernatant was discarded. The pellet was resuspended in 160 mM ammonium chloride and incubated for 3 min at room temperature. ADMSCs were isolated by mash filtration (70-┬Ám nylon mash filter) and centrifuged at 600├Ś g for 10 min. The pellet was suspended in a 75-cm2 flask (Nunc, Invitrogen) as the primary culture and incubated at 37Ôäâ under 5% CO2. Non-adherent cells were removed by replacing the medium. Cells were fed every 3 days with 10 mL of complete DMEM media. When this primary culture of ADMSCs reached 80% confluence, cells were harvested using 0.25% Trypsin-EDTA, and washed in 10 mL of phosphate-buffered saline (PBS). Cell viability was assessed by Trypan blue staining.
Identification of ADMSCs
Fluorescence-activated cell sorting (FACS) was used to analyze the surface markers of rat ADMSCs. After 3 passages, the cells were trypsinized and resuspended in DMEM containing 10% fetal bovine serum (FBS). Samples were counted, centrifuged, and resuspended in PBS. The cells were placed into eppendorf tubes at 1├Ś106 cells per 1.5 mL, washed twice with PBS, and incubated for 1 hour at room temperature with the following fluorescein isothiocyanate-conjugated antibodies (Peprotech Inc., USA) : anti-rat CD90 (1 : 200), anti-rat CD45 (1 : 200), and anti-rat CD11b (1 : 200). In control groups, the cells were incubated in PBS without antibodies. The samples were then washed twice with PBS and analyzed by FACS.
Chondrogenic differentiation of ADMSCs
Chondrogenic differentiation of ADMSCs was achieved using the StemPro┬« Chondrogenesis Differentiation kit (Invitrogen, USA). About 1.6├Ś107 ADMSCs were resuspended in 1 mL of pre-warmed complete chondrogenesis medium. Micromass cultures were generated by seeding 5-┬ÁL droplets of cell solution in the center of multi-well plates. After cultivating micromass cultures for 2 hour under high humidity, warmed chondrogenesis medium was added to cultured vessels and incubated at 37Ôäâ and 5% CO2 for 14 days.
Nucleus pulposus (NP) cells isolation for co-culture
We carefully extract intervertebral disc from SD rat tail and hold the annulus and nucleus, separated as much as possible. Harvested nucleus pulposus (NP) were washed in Hank's Balanced Salt Solution (HBSS : Gibco, Invitrogen) and diced the sample with sterilized tools and washed twice with HBSS. The diced tissues were digested in pronase solution [5% FBS (Gibco, Invitrogen), 0.2% pronase (Calbiochem, USA) in PBS (pH 7.4)] for 1 hour at 37Ôäâ. Pellet was collected by centrifugation for 10 min at 600├Ś g, then the supernatant was discarded. After that the pellet was resuspended in collagenease-P solution [5% FBS, 0.02% collagenase-P (Roche, USA) in PBS (pH 7.4)] and digestion for 1 h at 37Ôäâ. Cells were collected by centrifugation for 5 min at 600├Ś g, then the supernatant was discarded either. The pellet was resuspended in HBSS and washed for 5 min, and cells were collected by centrifugation for 10 min at 600├Ś g, and the supernatant was discarded. NP cells placed in a 75-cm2 flask (Nunc, Invitrogen) as the primary culture and incubated at 37Ôäâ under 5% CO2. Non-adherent cells were removed by replacing the medium. Cells were fed every 3 days with 10 mL of DMEM media.
Co-culture of chondrogenesis-differentiated ADMSCs and NP cells
We used a 6-well co-culture system (Nunc, USA). Due to the low-protein binding property of PET membranes, wide cotact area and numerous micro pore cell, culture inserts are especially suited for co-culture. Chondrogenesis-differentiated ADMSCs were placed on the bottom layer, while the nucleus pulposus cells were placed on the upper layer. The cells were incubated for 14 days and the medium was changed every 3 days ( Fig. 1).
Immunohistochemical evaluation
After 2 weeks of co-culture, all cells were fixed with acetone for 10 min. The cells were washed 3 times with pH 7.6 Tris-buffered saline (TBS), and then blocked with TBS containing 5% bovine serum albumin and 0.1% Triton X-100 for 1 hour at room temperature. After incubating overnight with primary antibody at 48Ôäâ, the sections were washed 3 times with TBS and incubated with fluorescent-conjugated secondary antibody (1 : 1000; Invitrogen, USA). Polyclonal antibodies against anti-aggrecan (1 : 200; Abcam, UK) and collagenase type II (1 : 200; Abcam, UK) were used as primary antibodies. Stained cells were observed under a fluorescence microscope (Carl Zeiss Meditec, Germany). Appropriate controls, including omission of either primary or secondary antibodies, were included to confirm that the primary antibodies produced specific staining.
RT-PCR
For RNA isolation, media was aspirated from the dishes and the cells were washed with 1 mL of diethylpyrocarbonate-treated water. One milliliter of TRIzol┬« reagent was added to each dish. Homogenized samples were incubated for 5 min at room temperature, and 200 ┬ÁL of chloroform was added per 1 mL of TRIzol┬« reagent by pipetting. The samples were shaken vigorously for 15 s and centrifuged at 12000├Ś g at 4Ôäâ for 10 min. The aqueous phase was transferred to a fresh tube, and 500 ┬ÁL of isopropyl alcohol was added per 1 mL of TRIzol┬« reagent. The sample was incubated at room temperature for 10 min to allow RNA to precipitate, and then centrifuged at 12000├Ś g for 10 min at 4Ôäâ. The RNA was washed with 1 mL of 75% EtOH and centrifuged at 7500├Ś g for 5 min at 4Ôäâ. The RNA pellet was briefly dried, resuspended in RNase-free water, and incubated for 10 min at 60Ôäâ. The cDNA synthesis reactions were incubated at 55Ôäâ for 50 min [cDNA mixture : 2 ┬ÁL of 10├Ś RT buffer, 4 ┬ÁL of 25 mM MgCl 2, 2 ┬ÁL of 0.1 M dithiothreitol, 1 ┬ÁL of RNaseOUTÔäó (40 U/┬ÁL), 1 ┬ÁL of SuperscriptÔäó III (200 u/200 ┬ÁL)]. The mixture was then incubated at 85Ôäâ for 5 min and placed on ice. One microliter of RNase H was then added and incubated for 20 min at 37Ôäâ. For PCR, sense and anti-sense primers, template DNA, and distilled water were used. Primers were used for ╬▓-actin, aggrecan, collagen type II, SOX9, and HIF-1╬▒ ( Table 1).
Western blotting
ADMSCs were harvested after 3 passages. Chondrogenesis-differentiated ADMSCs and co-cultured chondrogenesis-differentiated ADMSCs were harvested after 14 days of treatment. Proteins were extracted by homogenization in 100 ┬ÁL of lysis buffer containing 20 mM Tris pH 7.4, 50 mM NaCl2, 1% Triton X100, and protease inhibitors (Intron, Korea). Debris was cleared by centrifugation, and aliquots of the supernatants were stored at -80Ôäâ. Samples were assayed for protein concentration using bicinchoninic acid assay (Bio-Rad, Richmond, CA, USA). After heating at 100Ôäâ for 5 min in 5├Ś sample buffer, equal amounts of denatured protein (30 ┬Ág) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels. Proteins were transferred onto polyvinylidene fluoride membranes at 20 V for 30 min by using a Semi-Dry Trans-Blot apparatus (Bio-Rad). After incubating for 1 hour at room temperature with blocking solution (0.4% Tween-20 and 5% dry milk in PBS), membranes were incubated overnight with primary antibodies against aggrecan (1 : 200; Abcam, UK) or collagen type II (1 : 200; Abcam, UK). An antibody against ╬▓-actin (1 : 10000; Sigma, St. Louis, MO, USA) was used as a control for equal loading. After washing with TBS/0.1% Tween-20, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (Sigma, USA) and developed using enhanced chemiluminescence reagents (Pierce Biotechnology, USA).
Statistical analyses
The SPSS Version 12.0 (SPSS Inc., Chicago, IL, USA) statistical package was utilized for statistical analyses. Data was represented as mean┬▒standard deviation and repeated measure analysis of variance with post-hoc analysis was used to analyze the differences in radiological measurements at 2, 4, and 6 weeks after cell injection in NC, IN, and TS segments. p values less than 0.05 were considered statistically significant.
RESULTS
Culture of isolated ADSCs in monolayer and identification of ADMSCs
ADMSCs cultured in monolayer had a fibroblast-like shape under an inverted phase-contrast microscope. FACS analysis showed that rat ADMSCs expressed a number of specific markers of stem cells : CD90, CD45, and CD11. Therefore, we concluded that rat ADMSCs were positive for CD90 and negative for CD45 and CD11b ( Fig. 2).
Chondrogenic differentiation and immunohistochemical evaluation of ADMSCs
We determined the chondrogenic differentiation status of ADMSCs by inverted phase-contrast microscopy. Immunohistochemical analysis revealed positive staining for aggrecan and collagen II ( Fig. 3).
RT-PCR
The results of RT-PCR indicated that ADMSCs treated with TGF-╬▓3 showed a significant increase in the gene expression of aggrecan, collagen II, and Sox-9 over untreated ADMSCs. Furthermore, co-cultured ADMSCs treated with TGF-╬▓3 had further elevated expression of these genes compared to the ADMSCs treated with TGF-╬▓3 alone ( Fig. 4). Therefore, co-cultured ADMSCs treated with TGF-╬▓3 showed the maximum expression of differentiation markers among these samples.
Western blotting
Western blotting showed results similar to RT-PCR. Moreover, co-cultured ADMSCs treated with TGF-╬▓3 expressed more extracellular matrix compared with ADMSCs alone or ADMSCs treated with TGF-╬▓3 ( Fig. 5).
DISCUSSION
Adipose tissue is an abundant, accessible, and replenishable source of adult stem cells. The ADMSCs are multipotent and differentiate along the adipocyte, chondrocyte, myocyte, neuronal, and osteoblast lineages. ADMSCs have potential applications for the repair and regeneration of acute and chronically damaged tissues. Most importantly, a comparative analysis of the MSCs obtained from the bone marrow, adipose tissue, and umbilical cord clearly showed that adipose-derived cells were not different from the MSCs derived from other tissues with regard to morphology, immune phenotype, success rate of MSC isolation, colony frequency, and differentiation capacity 3). Therefore, this suggests that adipose tissue is the most attractive source for MSCs for researchers and clinicians in nearly all medicinal subspecializations 2). In our study, we used three-dimensional cultured ADMSCs which was confirmed their identity by FACS analysis. Some studies showed that TGF-╬▓-mediated induction protocols for MSCs in 3D culture resulted in a gene expression profile highly similar to that of native IVDs tissue. Furthermore it's molecular and histological appearance closer to fibrocartilage than hyaline articular cartilage 6). However, these common protocols for TGF-╬▓-mediated chondrogenesis seem to be most suitable for generation of a collagen type I-rich fibrocartilaginous tissue and has tendency towards a molecular phenotype and morphological features of hyaline cartilage. Therefore, these methods may need to be adapted to reach transcription levels close to IVD tissue. In our study, to elucidate the effect of TGF-╬▓-mediated chondrogenesis and co-culture ADMSC with NP cells, we performed comparative study for cultured ADMSCs, ADMSCs treated with TGF-╬▓ and co-cultured ADMSCs treated with TGF-╬▓ and accomplished immunohistochemical staining and extracellular matrix expression by PCR and western blotting. We found that all of them have nucleus pulposus-like activity and the expression of differentiation markers and extracellular matrix. Co-culture methods NP cell with ADMSCs treated with TGF-╬▓ was one of the modification methods to reach transcription levels close to IVD tissue. The previous studies have been reported the suitable cell population with the capacity to regenerate IVD tissue and acquisition methods of an unlimited cell source with an expression profile resembling that of native IVD tissue 6). Although promising, stem cell-based regenerative medicine remains a developing field. For disc regeneration treatment, further advances are required, and many issues await clarification. The one major issue is the biologic mechanism of MSC transplantation. A previous study reported that MSCs directly penetrate into damaged tissue and secrete or indirectly generate various cytokines that are useful for tissue regeneration, including nerve growth factor, neurotrophic factor, and vascular endothelial cell growth factor. Other studies have revealed that MSCs stimulate intrinsic stem cells in the tissue and differentiate target tissues 4). In this study, we revealed that the rat ADMSCs and rat ADMSCs treated with TGF-╬▓3 could be differentiated into functional NP-like cells. The rat ADMSCs and rat ADMSCs treated with TGF-╬▓3 expressed differentiation markers and extracellular matrix. Therefore, we expected that all of them were suitable for regeneration therapy following IVD degeneration. Furthermore, co-cultured ADMSCs treated with TGF-╬▓ was more effective to the regeneration of intervertebral disc than the other cells. The other major issue is clarification of the suitable cell population and environment. The pre-loading of biological signals to MSCs prior to implantation in order to "direct" MSC or host cell differentiation, such as using cytokines or genes, or co-culture, has been tested as a means to enhance stem cell therapy 5). Cells in the NP are heterogeneous, comprised of notochordal cells and chondrocyte-like nucleus pulposus cells. Notochordal cells in the nucleus pulposus have been proposed to act as "organizers" or behave as stem cells for IVD maintenance and repair 1). The origin, function, and characteristics of NP cells, and the signals required for NP cell differentiation, remain unclear. Some previous studies have attempted to stimulate MSCs to differentiate into chondrocyte-like or NP-like cells using cytokines or genes. For example, MSCs carrying a SOX9-expressing adenovirus were seeded into a synthetic poly-lactic acid polymer scaffold prior to implantation 8). Other studies have used co-culturing methods with differentiated cells that can determine the fate of MSCs. They revealed that adipose-derived rabbit MSCs can activate chondrocytic markers such as collagen II and aggrecan by NP tissues through soluble mediators 6). This study shows that ADMSCs treated with TGF-╬▓3 expressed more than ADMSCs alone. Furthermore, ADMSCs treated with TGF-╬▓3 and co-cultured with NP cell expressed more differentiation markers and extracellular matrix than ADMSCs treated with TGF-╬▓3. Therefore, the cellular microenvironment (including the co-culture status), was found to be the most important factor, as chondrocyte-specific gene expression and extracellular matrix production were the maximum greatest in our co-culture system. The limitation of this study is that the design of study was in vitro type. Furthermore, evaluation parameter of this study has not totally representative for intervertebral disc regeneration effect. Therefore, further study should be combined in vitro and vivo study with detailed design.
CONCLUSION
ADMSC treated with TGF-╬▓3 maybe an attractive source for regeneration therapy in degenerative IVD. These findings may also provide evidence into the physiologic mechanism of MSC therapy for the degeneration of IVD in vivo.
Acknowledgements
The Authors wish to thank Basic Science Research Program Through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number : 2009-0068925).
References
1. Hunter CJ, Matyas JR, Duncan NA : The notochordal cell in the nucleus pulposus : a review in the context of tissue engineering. Tissue Eng 2003, 9 : 667-677,   2. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al : Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006, 99 : 1285-1297,    3. Kern S, Eichler H, Stoeve J, Kl├╝ter H, Bieback K : Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24 : 1294-1301,   4. Le Visage C, Kim SW, Tateno K, Sieber AN, Kostuik JP, Leong KW : Interaction of human mesenchymal stem cells with disc cells : changes in extracellular matrix biosynthesis. Spine (Phila Pa 1976) 2006, 31 : 2036-2042,   5. Leung VY, Chan D, Cheung KM : Regeneration of intervertebral disc by mesenchymal stem cells : potentials, limitations, and future direction. Eur Spine J 2006, 15( Suppl 3):S406-S413,   6. Li X, Lee JP, Balian G, Greg Anderson D : Modulation of chondrocytic properties of fat-derived mesenchymal cells in co-cultures with nucleus pulposus. Connect Tissue Res 2005, 46 : 75-82,   7. Richardson RM, Barbaro NM, Alvarez-Buylla A, Baraban SC : Developing cell transplantation for temporal lobe epilepsy. Neurosurg Focus 2008, 24 : E17,  8. Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, et al : The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials 2006, 27 : 4069-4078,   9. Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al : Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc : a potential therapeutic model for disc degeneration. Biomaterials 2003, 24 : 3531-3541,   10. Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, et al : An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine (Phila Pa 1976) 2003, 28 : 548-553,   11. Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W : Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells 2005, 23 : 403-411,  
Fig. 1
Schematic diagram shows co-culture system. Insert has low-protein binding property of PET membranes, wide contact area and numerous micro pore. ADMSC : adipose-derived mesenchymal stem cells, NP : nucleus pulposus. 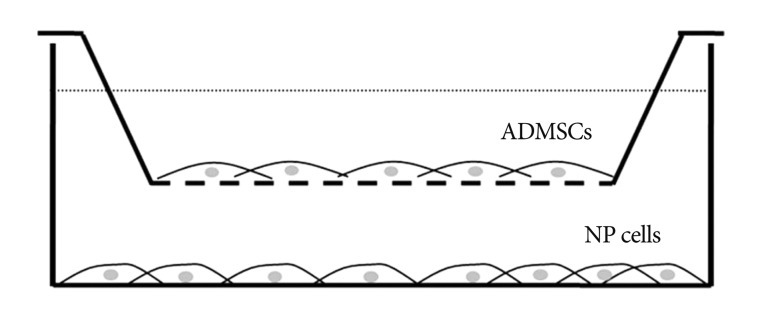
Fig. 2
FACS analysis shows that rat ADMSCs express high levels of CD90/thy1 and low levels of CD45 and CD11b. FACS : fluorescence-activated cell sorting, ADMSCs : adipose-derived stem cells. 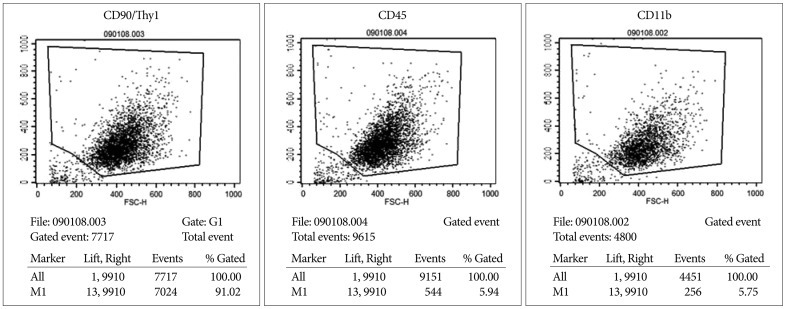
Fig. 3
Immunohistochemical analysis shows positive staining for aggrecan and collagen II (scale bar=100 ┬Ám). ADMSCs : adipose-derived stem cells, TGF-╬▓3 : transforming growth factor ╬▓3. 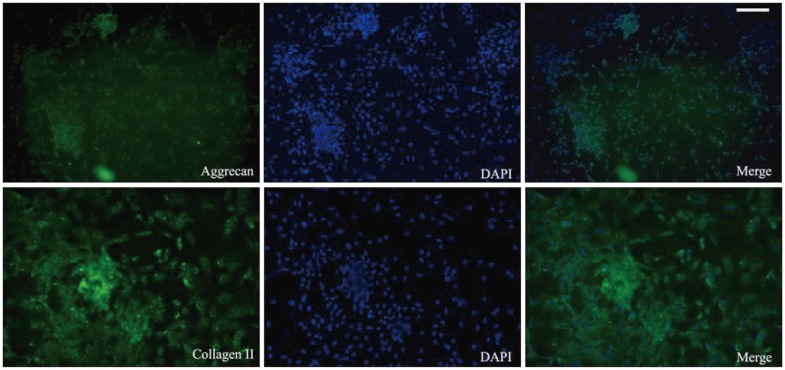
Fig. 4
Results of RT-PCR show that ADMSCs treated with TGF-╬▓3 displayed a significant increase in the gene expression of aggrecan, collagen II, and Sox-9 over untreated ADMSCs. Co-cultured ADMSCs treated with TGF-╬▓3 was further elevated compared with the ADMSCs treated only with TGF-╬▓3. 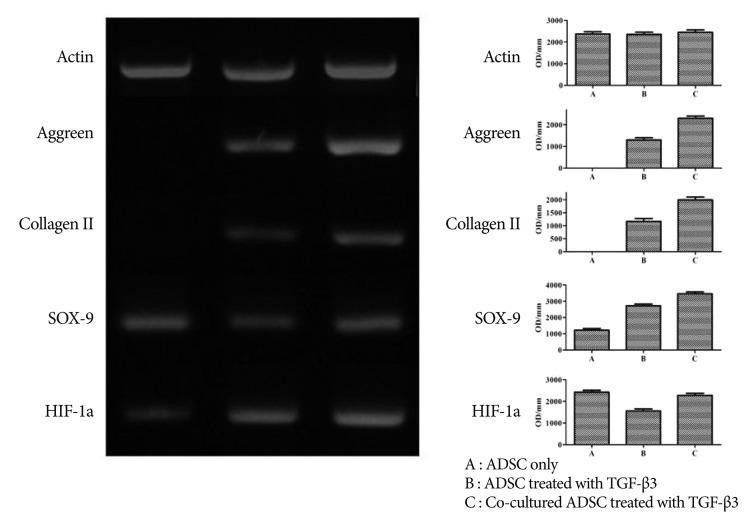
Fig. 5
Western blotting showed that co-cultured ADMSCs treated with TGF-╬▓3 had greater expression of extracellular matrix compared with ADMSCs alone or ADMSCs treated only with TGF-╬▓3. 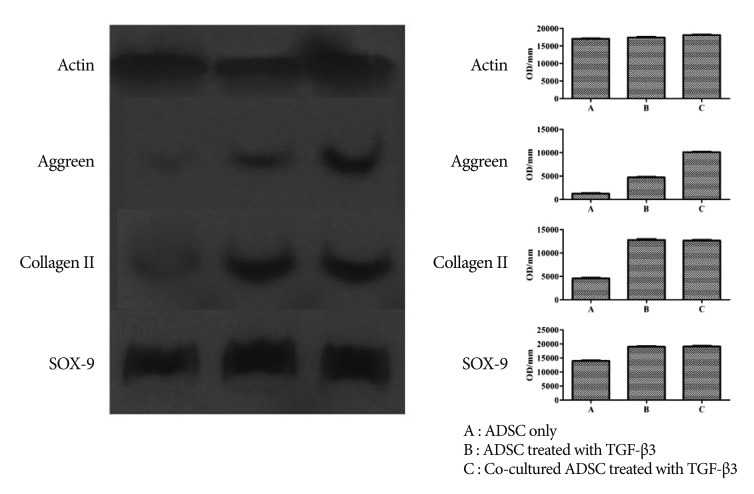
Table 1

|
|





















