Oh, Lee, Lee, Jung, Whang, and Brain Research Group: The Meaning of the Prognostic Factors in Ruptured Middle Cerebral Artery Aneurysm with Intracerebral Hemorrhage
Abstract
Objective
This study analyzed the relationship between prognosis and multiple clinical factors of ruptured middle cerebral artery (MCA) aneurysm with intracerebral hemorrhage (ICH), to aid in predicting the results of surgical treatment.
Methods
Enrolled subjects were 41 patients with ruptured MCA aneurysm with ICH who were treated with surgical clipping. Clinical factors such as gender, age, and initial Glasgow coma scale were assessed while radiological factors such as the volume and location of hematoma, the degree of a midline shift, and aneurysm size were considered retrospectively. Prognosis was evaluated postoperatively by Glasgow outcome scale.
Results
Age and prognosis were correlated only in the groups with ICH over 31 mL or ICH at the frontal lobe or sylvian fissure. When initial mental status was good, only patients with ICH on the temporal lobe had a better prognosis. If the midline shift was less than 4.5 mm, the probability of better prognosis was 95.5% (21 of 22). If the midline shift was more than 4.5 mm, the probability of poor prognosis was 42.1% (8 of 19). Patients with ICH less than 31 mL had higher survival rates, whereas if the ICH was more than 31 mL, 41.2% (7 of 17) had a poor clinical pathway.
Conclusion
Even if the initial clinical condition of the patient was not promising, by carefully examining and taking into account all factors, neurosurgeons can confidently recommend surgical treatment for these patients.
Key Words: Ruptured middle cerebral artery aneurysm · Intracerebral hemorrhage · Prognostic outcome.
INTRODUCTION
Middle cerebral artery (MCA) aneurysms are 20% of saccular aneurysms. They are occasionally found incidentally or because of a mass effect, but most are found when they rupture 8). After Dott 2) treated ruptured aneurysms with surgical methods, many treatment modalities have been developed and reported 17). When MCA aneurysms rupture, approximately 30-50% lead to a subarachnoid hemorrhage (SAH) combined with an intracerebral hemorrhage (ICH), with a mortality rate of 10-41% 4,16). The mechanism of ICH developed by aneurysmal rupture is still controversial but the most commonly accepted hypothesis is that the ICH develops when fibrin, hematoma, or the fibrosis of the arachnoid membrane obstructs the subarachnoid space, or an aneurysm attached to the pia ruptures 16). These ICHs are a risk factor for poor prognosis and surgical complications in ruptured MCA aneurysm patients. However, our clinical data suggested that patients with ruptured MCA aneurysms with ICH have a non-classical clinical course that requires further study. Therefore, authors analyzed the relationship between multiple clinical factors of ruptured MCA aneurysms with ICH and general prognosis, to help predict the results of surgical treatment.
MATERIALS AND METHODS
We retrospectively reviewed 68 patients with MCA aneurysm who underwent neck clipping after aneurysm rupture between January 2006 and December 2010. We excluded 27 patients who did not have accompanying ICH. The remaining 41 patients with accompanying ICH were evaluated. Aneurysm, SAH and ICH were diagnosed with computed tomography (CT) and conventional cerebral angiography. Of the 41 total patients, 27 were evaluated with preoperative angiography ( Table 1).
Clinical, Radiologic evaluation
Clinical status was evaluated with gender, age, and Glasgow coma scale (GCS). Clinical outcome was evaluated with the GOS 6). Patients with a GOS of 4-5 were considered to be a favorable outcome group. On contrary, patients with a GOS of 1-3 were considered to be an unfavorable outcome group. According to hematoma location, radiologic findings were classified into temporal, sylvian fissure, and frontal groups ( Fig. 1). Hematoma volume was measured with abc/2 methods. We also evaluated lesion lateralization, degree of midline shift, and aneurysm size.
Surgical methods and postoperative management
All patients were operared with a routine pterional approach. If severe brain swelling was found, duroplasty and decompressive craniectomy were performed. The medial transsylvian approach and lateral transsylvian approach were chosen appropriately in each situation with a goal for less brain retraction and less brain injury. All surgery was performed on the day of admission. Postoperative CT brain contrast perfusion images were obtained for all patients to evaluate perfusion values such as cerebral blood flow, cerebral blood volume, and mean transit time. According to each perfusion value, we performed osomotherapy with mannitol, or treated with a calcium channel blocker such as Nimodipine®. In case of vasospasm events, 3H therapy of hypertension, hypervolemia, hemodilution was performed.
Statistical analysis
All parameters were evaluated retrospectively. Prognostic factors were evaluated with a chi-square test, independent t-test, receiver operation characteristic (ROC) curve, McNemar test, and nonparametric chi-square test with significance defined as less than 0.05. All statistical analysis was performed with commercial software (Predictable Analysis Soft Ware, Version 18.0, IBM Inc.)
RESULTS
Age
Patients' mean age was 58.2 years. Patients aged 65 years and younger had a significantly better prognosis than those over 65 years ( p<0.05). Classifying the patient groups based on hematoma location showed that younger patients with ICH on the frontal lobe or sylvian fissure had a significantly better prognosis. Meanwhile, patients with ICH on the temporal lobe did not show any correlation between age and prognosis ( Table 2). By classifying the patients based on hematoma amount, its volume less than 31 mL had some correlation where younger age meant better prognosis. However, if the amount of hematoma was more than 31 mL, no significant correlation was noted between age and prognosis ( Table 3).
Gender
Among 41 patients, there were 11 male patients and 30 female patients. Of 11 male patients, one patient had an unfavorable outcome while 10 others had favorable outcome. On the other hand, of 30 female patients, 8 patients had an unfavorable outcome while 22 others had favorable outcome. By gender, the proportion of male patients in the favorable outcome group was higher than female patients, but this was not significant (p>0.05). When patients were analyzed based on location and volume of ICH, gender and clinical outcome were not significantly correlated (p>0.05).
Lateralization of brain lesion
Patients who had ICH in the dominant hemisphere were 23 which outnumbered those who had ICH on the non-dominant hemisphere (18/41). Of 23 patients with ICH in the dominant hemisphere, 5 had unfavorable outcome while 18 others had favorable outcome. In contrast, of patients with ICH in the non-dominant hemisphere, 4 had an unfavorable outcome while 14 had a favorable outcome. However, this was not significant (p>0.05).
Initial mental status
According to GCS score, patients were classified into three groups : a severe group with GCS 3-8, a moderate group with GCS 9-12, and a mild group with GCS above 13. Among the 41 patients with ICH, 5 were in severe group (12.1%), 7 were in the moderate group (12.1%) and 19 were in the mild group (46.3%). Furthermore, there was a statistical significance between the initial mental status and clinical outcome ( p<0.05). Based on hematoma location, clinical outcome was significantly more favorable in patients with better initial mental status when the hematoma was in the temporal lobe ( p<0.05). On the contrary, initial mental status and clinical outcome were not correlated in the patient group with ICH in the frontal lobe or sylvian fissure ( p>0.05) ( Table 4).
Aneurysm size
By aneurysm size, patients were classified into three groups : small, with an aneurysm less than 10 mm in diameter; large, with a diameter of 10-24 mm; and giant, with a diameter greater than 24 mm. The small group of 11 patients with a small aneurysm had two unfavorable outcomes (18.2%) and nine favorable outcomes (81.8%). Of 29 patients with a large aneurysm, 7 had an unfavorable outcome (24.1%) and 22 had a favorable outcome (75.9%). One patient with a giant aneurysm had a favorable outcome. However, aneurysm size and clinical outcome were not significantly correlated. No statistical relationship was seen among aneurysm size, presence of a midline shift, or initial mental status (p>0.05). Analysis of the relationship between hematoma volume and aneurysm size, showed an average volume of 18.64 mL for the small aneurysm group and 38.74 mL for the large aneurysm group. However no significant relationships were found.
Hematoma location
ICHs were divided into three groups based on location : 18 had frontal ICH, 11 had sylvian ICH (26.8%), and 12 had temporal ICH (29.3%). Of the three locations, the temporal ICH group had the most favorable clinical outcome (42.6%, 5/12) while in the frontal ICH, survival was 6.7%, and in the sylvian fissure ICH, it was 21.4%. However, no significant conclusions could be drawn.
Midline shift
Of the 41 patients, 24 were had a midline shift of the brain parenchyma, with a midline shift range of 3 mm to 15 mm. The average length of the midline shift was 7.5 mm. Patients with a poor prognosis showed a mean midline shift of 8.36 mm while those with a good prognosis had a mean midline shift of 3.3 mm, for a 5.06 mm difference ( p<0.05). Based on the ROC curve, sensitivity was 89% and specificity was 69% if the midline shift was around 4.5 mm. If the midline shift was less than 4.5 mm, the positive predictive value for patients with better prognosis was 95.5% (21 of 22) which suggested an authentically significant result. If the midline shift was more than 4.5 mm, the negative predictive value (the probability of poor prognosis) was only 42.1% (8 of 19) ( Table 5). The accuracy of the ROC curve was moderate, as the area under the curve (AUC) was 0.806.
Preoperative hematoma volume VS post-operative residual hematoma volume
If the preoperative hematoma volume was large, the clinical outcome was poor ( p<0.05). By applying the ROC curve, the highest sensitivity was 78% and highest specificity was 69% for patients with a hematoma volume of 31 mL. When compared the clinical outcome of patients with 31 mL of hematoma volume, the positive predictive value (probability of favorable prognosis with hematoma volume less than 31 mL) was very high, at 91.7% (22/24). However, the negative predictive value (probability of poor prognosis for those with hematoma volume more than 31 mL) was relatively low as 41.2% (7/17) ( p<0.05) ( Table 6). The accuracy of the ROC curve was moderate with an AUC of 0.740. Of 17 patients with an ICH volume higher than 31 mL, 11 patients had their hematoma evacuated and reduced to 20 mL. Patients with lower residual hematoma showed a better clinical outcome than those with a larger residual hematoma. However, even if the residual hematoma volume was less than 20 mL, those with a minimal hematoma volume at the initial stage showed a better prognosis than those with a hematoma that was surgically removed with remnant hemorrhage ( p<0.05).
DISCUSSION
Niikawa et al. 13) reported that intracranial pressure elevation and brain injury in ruptured MCA with ICH patients are directly correlated with hematoma size and location 15,18). And, patients with ruptured MCA with associated ICH were reported to have a high mortality rate 8). ICH is more common with distal anterior cerebral artery aneurysms. Since the frequency of ruptured distal anterior cerebral artery aneurysm is low, aneurysms on the MCA or anterior communicating artery are the most common for accompanying ICH 8). When there were ruptured MCA aneurysms, Niikawa et al. 13) reported that 36% of SAH patients also had ICH. According to the domestic report, Lee et al. 9) reported a slightly higher rate. Nowak et al. 14) reported a mortality rate of 36% for ruptured aneurysm with ICH, with domestic statistics on mortality rate slightly lower, at 2.3-8.3% 10). This study excluded patients with absence of brain stem function and perfusion defect; thus, the exact mortality rate was not determined. The most common causes of death are sudden elevation of intracranial pressure, and a midline shift of brain parenchyma from a mass effect 1,15). It is generally believed hat ruptured MCA aneurysms with ICH will have a worse prognosis than ruptured MCA aneurysms with SAH only 18). The larger the hematoma volume, the more mass effect on the adjacent brain parenchyma. Hence, more severe brain damage is inevitable. Our study analyzed the correlation among multiple parameters such as gender, initial mental status, age, and radiologic factors and lesion lateralization, hematoma volume, location, midline shift, postoperative volume of residual hematoma, and prognosis. No significant correlation was seen among the parameters of gender, lesion lateralization, or clinical outcome. According to the previous literature, Kazumata et al. 7) and Kim et al. 8) also analyzed the relationships among the same parameters in conjunction with for clinical outcome, and found no significant correlations. Younger patients had a better clinical outcome than older patients in this study. Positive correlation was seen for age and prognosis for ICH in the frontal lobe or sylvian fissure, but no significant conclusions were drawn for ICH in the temporal lobe. When we set the volume of hematoma as a fixed parameter to determine the patient's prognosis, younger age patients had more favorable clinical outcome in the study group with minimal volume of hematoma as it was proven statistically significant ( p<0.05). On the other hand, one cannot guarantee that elderly SAH patients with a large amount of ICH on the temporal lobe will all have poor clinical outcome. A poor prognosis must not be the first and absolute parameter when making the decision for the surgical treatment. The better the initial mental status at admission, the more favorable clinical outcome was observed ( p<0.05) as this was mentioned in the previous literature 5,12). In this study, the positive correlation between good initial mental status and a good prognosis was statistically valid only in the patient group with ICH located on the temporal lobe. As many previous reports have discussed, there was the direct correlation between the prognosis of ruptured aneurysm with ICH and the initial mental status 3,11). A new design of study with a larger study population is necessary to re-analyze the data statistically in the near future. There was no definite difference in the clinical outcome when the determining factor was the ICH location. However, Lee et al. 9) reported that patients with ICH located on the temporal lobe showed a more favorable prognosis. Whereas Kim et al. 8) reported that the same favorable result was observed from the patients with the ICH located in the sylvian fissure. Although results were not statistically significant, our study showed that those with ICH on the temporal lobe showed a better clinical prognosis. A midline shift on brain CT was another critical factor to determine a patient's prognosis due to its mass effect. When we set the cut-off value for a midline shift as 4.5 mm, 95.5% of patients had a favorable clinical outcome when the midline shift was less than 4.5 mm. However, when it was more than 4.5 mm, 42.1% patients showed a poor clinical outcome, although it comprised a relatively low percentage in the total population. In a similar manner, if we set the hematoma volume fixed to 31 mL, specificity and sensitivity was the highest. If the hematoma volume was less than 31 mL, 91.7% of the patients showed a better clinical outcome. However, if the hematoma volume was more than 31 mL, a relatively small proportion of patients (41.2%) had unfavorable outcome. Therefore, it was expected that the patients with minimal hematoma volume showed a more favorable surgical outcome. Nevertheless, even if the patients had a larger volume of hematoma with a midline shift, this should not be an absolute criterion to determine a patient's prognosis to give up the surgical treatment. In summary, the less the residual hematoma volume, the better the patient prognosis, as the hemorrhage itself significantly influenced to cause cerebral edema, vasospasm, and multiple brain injury. This study also demonstrated that the patients with less residual hematoma volume showed a more favorable prognosis. Yet, it was obvious that the patients with minimal residual hematoma volume showed a less favorable prognosis than those without ICH. The surgical intervention definitely prevents the secondary brain injury. Nonetheless, it is important to consider that initial brain injury was more critical in those with ICH than those without.
CONCLUSION
This study demonstrated that predictors of a good prognosis for ruptured MCA aneurysm were under 65 years old, had good initial mental status, a preoperative hematoma volume below 31 mL, and a midline shift of less than 4.5 mm. These predictors were important in determining the prognosis of surgical outcome. However, also important to note was that patients without good prognostic factors did not necessarily proceed to poor surgical outcome, as this probability was relatively low. Thus, neurosurgeons are encouraged to recommend an early intervention of surgical treatment even for patients who show poor prognosis, as many interrelated factors influence the clinical outcome.
Acknowledgements
This work was supported by Yonsei University, Wonju College of Medicine research fund (YUWCM-2011-45).
References
1. Davies DM : Diagnosis and treatment ot stupor and coma. Med World 1959, 91 : 9-13,  2. Dott NM : Intracranial aneurysms : cerebral arterioradiography; surgical treatment. Edinb Med J 1933, 40 : 219-234,
3. García-Ruiz PJ, Garrido Martínez NE, Guerrero Solá A : [Spontaneous intracerebral hemorrhage. Epidemiology, evolution and prognosis in a series of 73 cases]. Rev Clin Esp 1988, 182 : 356-359,  4. Graf CJ, Nibbelink DW : Cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Report on a randomized treatment study. 3. Intracranial surgery. Stroke 1974, 5 : 557-601,   5. Horiuchi T, Tanaka Y, Takasawa H, Murata T, Yako T, Hongo K : Ruptured distal middle cerebral artery aneurysm. J Neurosurg 2004, 100 : 384-388,   6. Jennett B, Bond M : Assessment of outcome after severe brain damage. Lancet 1975, 1 : 480-484,   7. Kazumata K, Kamiyama H, Yokoyama Y, Asaoka K, Terasaka S, Itamoto K, et al : Poor-grade ruptured middle cerebral artery aneurysm with intracerebral hematoma : bleeding characteristics and management. Neurol Med Chir (Tokyo) 2010, 50 : 884-892,   8. Kim H, Shim YB, Hwang HS, Choi JJ, Kim SM, Park YK, et al : The clinical analysis of bleeding pattern in patients with ruptured middle cerebral artery aneurysm. J Korean Neurosurg Soc 2001, 30 : 699-704,
9. Lee KC, Park HS, Joo JY, Jin BH : Overall management outcome of patients with ruptured intracranial aneurysm during a 2-year period. J Korean Neurosurg Soc 1995, 24 : 1030-1036,
10. Lee WC, Choi CH : Prognostic factors of ruptured middle cerebral artery aneurysm with intracerebral hematoma. J Korean Neurosurg Soc 2001, 30 : 91-98,
11. Manno EM, Meyer FB : Prognosis after intracerebral hemorrhage. J Neurosurg 2008, 108 : 1170-1171,   12. Morgan MK, Mahattanakul W, Davidson A, Reid J : Outcome for middle cerebral artery aneurysm surgery. Neurosurgery 2010, 67 : 755-761; discussion 761,   13. Niikawa S, Kitajima H, Ohe N, Miwa Y, Ohkuma A : Significance of acute cerebral swelling in patients with sylvian hematoma due to ruptured middle cerebral artery aneurysm, and its management. Neurol Med Chir (Tokyo) 1998, 38 : 844-848; discussion 849-850,   14. Nowak G, Schwachenwald D, Schwachenwald R, Kehler U, Müller H, Arnold H : Intracerebral hematomas caused by aneurysm rupture. Experience with 67 cases. Neurosurg Rev 1998, 21 : 5-9,   15. Plum F, Posner JB : The diagnosis of stupor and coma. Contemp Neurol Ser 1972, 10 : 1-286,  16. Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O : Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg 1997, 87 : 170-175,   17. Suzuki J, Yoshimoto T, Kayama T : Surgical treatment of middle cerebral artery aneurysms. J Neurosurg 1984, 61 : 17-23,   18. Tapaninaho A, Hernesniemi J, Vapalahti M : Emergency treatment of cerebral aneurysms with large haematomas. Acta Neurochir (Wien) 1988, 91 : 21-24,  
Fig. 1
Type of intracerebral hemorrhage. Axial CT scans, preoperation. A : Temporal type. B : Frontal type. C : Sylvian fissure type. 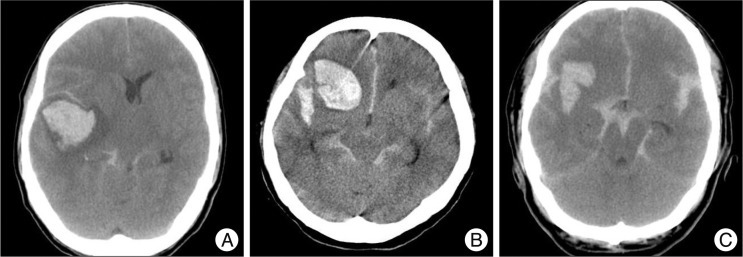
Table 1
Demographic statistics of ruptured MCA, aneurysm with ICH 
Table 2
Age and clinical outcome according to location 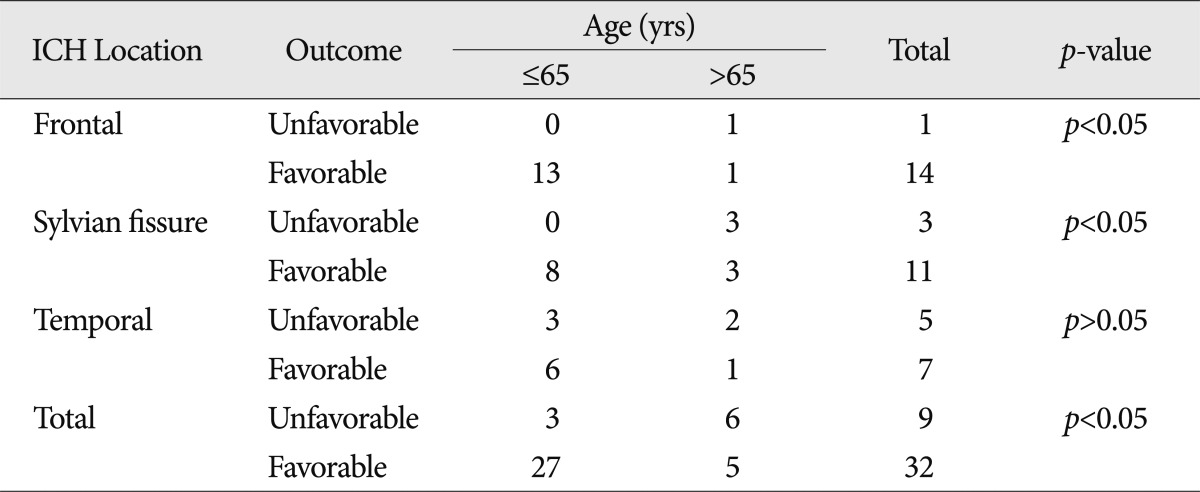
Table 3
Age and clinical outcome according to hematoma volume 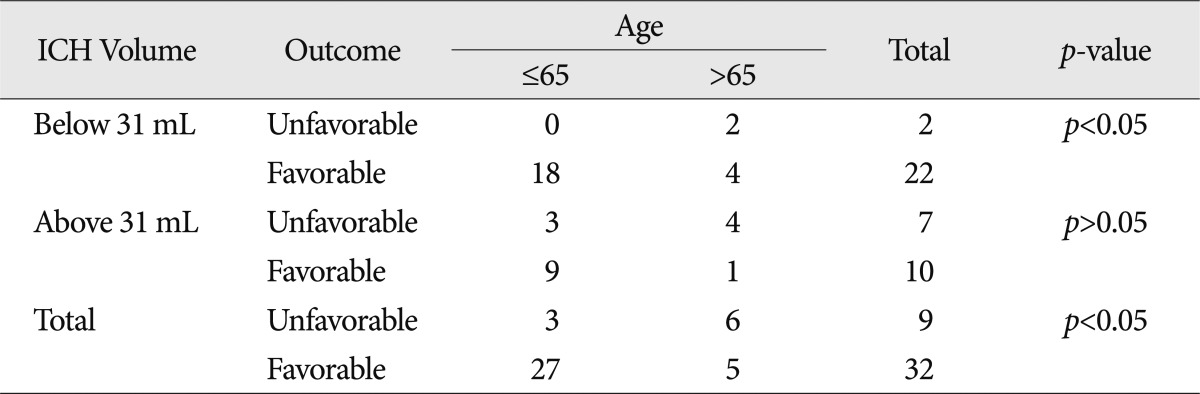
Table 4
Initial mental status and outcome 
Table 5
Midline shift and clinical outcome 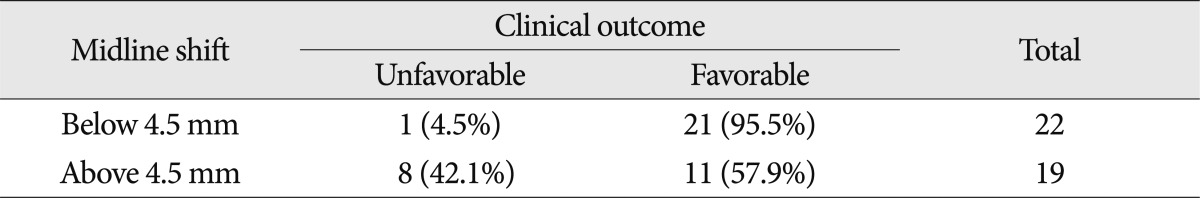
Table 6
Hematoma volume and clinical outcome 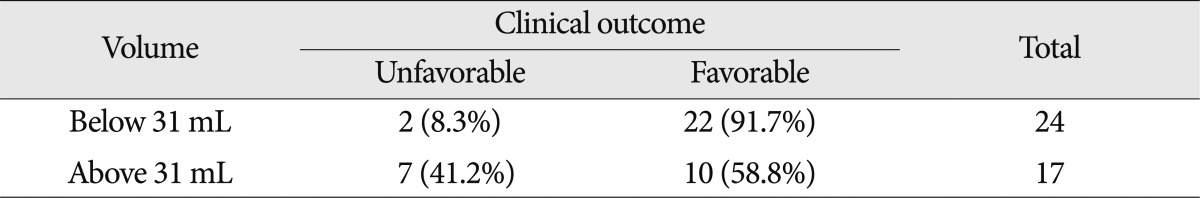
|
|






















