Tojima: A Tale of the Tail : A Comprehensive Understanding of the ŌĆ£Human TailŌĆØ
Abstract
Humans do not have tails; however, a congenital anomaly named ŌĆ£human tailŌĆØ has been recognized since old times. In contrast with its impactful name, the anomaly itself is not fatal, and thus it has not been considered as a clinically serious symptom. However, many case reports suggested that retention of ŌĆ£the tailŌĆØ is closely associated with spinal cord malformation and should be treated with care by neurosurgeons. Therefore, this review summarizes our knowledge regarding the anatomy, function, and development of the tail as a general structure in mammals. Learning the basic knowledge regarding tail anatomy and development would help clinicians to understand the ŌĆ£human tailŌĆØ more concisely and to select more appropriate examinations or treatments in relation to this congenital anomaly.
Key Words: Congenital abnormality ┬Ę Somites ┬Ę Morphogenesis ┬Ę Spine.
INTRODUCTION
We, humans, basically do not have tails. However, some clinicians would claim that there is a congenital anomaly named ŌĆ£human tailŌĆØ. This impressive anomaly has been occasionally reported in clinical case reports, but the anomaly seemed not to be regarded as a serious symptom. This is because the human tail itself is not fatal. Thus, the solid definition of this anomaly has not been established, and the cause has not been clarified yet. But many case reports regarding the human tail showed that this anomaly is highly associated with spinal dysraphism (over 40% of reported cases since 1880s [ 32]); neurosurgical treatments were required in such cases. Spinal dysraphism is not life threatening, but severe forms such as myelomeningocele, meningocele, lipoma, or tethered cord syndrome, are troublesome anomalies which could affect the patientsŌĆÖ quality of life. This anomaly has been reported all over the world including Asian countries. This review aimed to provide basic knowledge about the tail anatomy and its development for clinicians to understand this congenital anomaly ŌĆ£human tailŌĆØ substantially. Although humans basically do not have tails, clinicians may still obtain some benefits to better understand the purpose and structure of tails in other mammals. In fact, learning the basic knowledge regarding tail anatomy and development would help clinicians to understand the congenital anomaly, ŌĆ£human tail,ŌĆØ more concisely and to select more appropriate examinations or treatments.
WHAT IS A TAIL?
In vertebrates, a tail is generally defined as an elongated trunk that is posterior to the anus (or the cloaca). A tail is structurally homologous to the trunk but does not contain a body cavity; it includes musculoskeletal elements (caudal vertebrae and muscles) and neurovascular tissues that are associated with the muscles that move the tail. Tail length varies greatly among mammal species and even among different conspecific local populations [ 12, 35]. It is one of the most important morphological features that reflect the habitat, ecology, and evolutionary processes associated with a given species.
Anatomy of tails
The musculoskeletal system of the tail is presented in Fig. 1. The caudal vertebrae are part of the skeletal system that maintains the tail, which articulates posterior to the sacrum [ 28]. The morphology of the caudal vertebrae differs between the proximal and distal regions [ 2, 3, 13, 22, 29]. The proximal caudal vertebra, the morphology of which is similar to that of other vertebrae, provides attachments to the caudal musculature or maintains their muscular bellies [ 22, 29]. These vertebrae articulate with one another via their zygapophyses and intervertebral discs, as do other vertebrae. In contrast with the proximal caudal vertebrae, the distal caudal vertebrae are simply shaped in a vaguely hexagonal column. They possess neither processes nor arches and articulate only with the intervertebral discs. These morphological differences between the proximal and distal caudal vertebrae might affect tail mobility. The zygapophyses involved in articulation in the proximal region of the tail permit a certain degree of sagittal flexion but resist movement in other directions [ 26, 27, 33]. Conversely, articulation only via the intervertebral discs in the distal region allows movement in almost all directions [ 22]. Among the taxonomic groups of species that contain tails with varying lengths, the number of caudal vertebrae tends to vary with tail length [ 11, 22, 29]. The caudal musculature contains dorsal caudal extensors and abductors and ventral caudal flexors and pelvocaudal muscles. The dorsal caudal muscles are especially considered to be elongated portions of the erector spinae muscles. These structures contribute to the movement of the tail [ 22, 30]. The caudal musculature in tailed primates exists on both the ventral and dorsal sides, as in other mammals, and allows the tail to be flexed, extended, and abducted. The caudal muscles tend to insert more proximally in shorter-tailed primate species. The number of caudal muscles, as well as the number of vertebrae, tends to be reduced in shorter-tailed species [ 30].
Function of tails
In primates, tails play many important roles. Some New World monkeys (e.g., spider and howler monkeys) have prehensile tails and use them as a ŌĆ£fifth handŌĆØ; they can even suspend themselves by their tails. Most primates, however, do not have prehensile tails. Like in most other arboreal mammals, the tail in primates is generally used as a balancer, as supported by experimental studies, to increase stability in the unstable arboreal environment [ 17]. Indeed, when an individual walks or leaps from branch to branch, the tail whips left and right or rotates. Among the Old World monkeys, arboreal species tend to have longer tails than other species, implying that the need for balance is greater in arboreal species. Some tails also function in social communication [ 1, 4, 14, 16, 21, 23]. For example, tail carriage indicates the rank of a male within the troop hierarchy in some cercopithecoid species, such as the savanna monkey ( Cercopithecus aethiops). The alpha male has the highest rank and carries his tail in a sigmoid manner, while the beta male has the second-highest rank and carries his tail parallel to the ground. Similar signaling has been reported in the Japanese macaque ( M. fuscata). Tails may also be used for communication between male and female primates. While observing a troop of wild crab-eating macaques ( M. fascicularis) in Thailand, I noted some females holding their tails high and showing their vaginas to the alpha male. Immediately after the alpha male saw a female displaying this posture, he mated with her.
EVOLUTION OF TAIL LOSS IN HUMANS
It is striking that an organ with such multiple functions has become completely lost in hominoids (so-called apes), including humans. The tail-reduction process during evolution remains a mystery owing to incomplete fossil records. A common ancestral species of tailed cercopithecoids (Old World monkeys) and tail-less hominoids is Aegyptopithecus zeuxis. This species possessed a distinct distal portion of the caudal vertebra. Thus, we had an ancestor approximately 33 million years ago (Ma) with a long tail [ 10]. However, more recent ancestral hominoid species ( Proconsul heseloni, approximately 18 Ma, and Nacholapithecus kerioi, approximately 15.5 Ma) had already lost their tails completely [ 19, 20, 34]. Our ancestors lost their tails during an almost 20-million-year period, between the Oligocene and Miocene epochs. Unfortunately, no fossils connecting these ancestral species have ever been found ( Fig. 2).
TAIL MORPHOGENESIS DURING EMBRYONIC DEVELOPMENT
Axial extension and termination
The development of the tail is presented in Fig. 3. During embryonic development in amniotes (tetrapod vertebrate species that possess the amnion during development : reptiles and mammals), the body axis elongates and pairs of somites are generated along the axis. These somites are the primordia of the vertebrae and the erector spinae muscles. Somitogenesis in the caudal region proceeds in the same manner with a more anterior trunk : somites are generated sequentially from the presomitic mesoderm along the neural tube [ 18, 24]. When the somites are added, Fgf/Wnt proteins are secreted at the tip of the tail (tail bud). The Fgf/Wnt proteins are antagonistic to retinoic acid (RA) and are present in greater quantity than RA during axial elongation. When the axial elongation terminates, however, these two opposing conditions reverse and the amount of RA at the tail bud increases. New somite formation then stops [ 6, 25]. While the body axis elongates, Hox genes are at work, and variations in their expression characterize the body axis; they determine which embryonic portions become cervical, thoracic, lumbar, and sacrocaudal parts of the body. Studies utilizing mouse models revealed that posterior Hox genes (paralog group 10 to 13) are involved in the determination of tail length in mammals [ 5]. Another study demonstrated that the number of lumbar and sacral vertebrae was altered in double-knockout Hoxa10 and Hoxd11 mice [ 9]. Hoxb13 is also related to tail elongation in mice [ 36]. Another study revealed that a Hoxb13 knockout mouse had a longer tail and a greater number of caudal vertebrae than normal individuals [ 7].
Tail reduction
Previous studies regarding tail development have suggested that the timing of axial elongation termination is an important developmental event for tail length determination. However, a very recent study showed that another developmental event, tail reduction, is essential in determining the final tail length [ 31]. It is known that the once-formed embryonic tail diminishes and is completely lost during the human embryonic period. However, until recently, the detailed developmental process behind this event has not been clarified. The authors of a recent study used human embryonic specimens to study the transition of the number of somites and clarified that the caudal somites radically decrease in number immediately after the termination of axial elongation [ 31]. The cellular behavior and genes involved in this tail reduction have not yet been clarified, but the transition may involve apoptosis [ 8].
IS ŌĆ£HUMAN TAILŌĆØ A REAL TAIL?
The ŌĆ£human tailŌĆØ is not a fatal anomaly and is usually surgically removed by ablation; however, there have been many instances where the ablations were not treated with care, resulting in complications. Some studies have reported that this anomaly tends to be associated with spinal anomalies, but previous case reports were summarized only partially and locally, and pathological patterns including associated anomalies were not fully understood. Moreover, the definition of the ŌĆ£human tailŌĆØ itself was inconsistent and its classification varied [ 32]. The causes of this anomaly have not been studied carefully; it was believed to be a residual embryonic tail for more than 100 years. More specifically, it was believed that there is a region so-called caudal filament which does not contain any somites at the tip of the human embryonic tail [ 15]. Harrison [ 15] claimed that the human tail is caused from mal-reduction of the caudal filament. However, a very recent study using 3D reconstruction of human embryonic structure has shown that somites exists until the tip of the tail and the caudal filament is not observed in the human embryonic tail [ 31]. This finding denied the 100-year-old HarrisonŌĆÖs hypothesis regarding the cause of the human tail. Another study analyzed the case reports regarding the human tail and revealed that the cause of the anomaly is multiple though their appearances are similar. The study analyzing 195 cases of ŌĆ£human tailŌĆØ from 1900 to the present revealed that it can be classified into four types, based on its structure and location [ 32]. If the tail contains bones or cartilages inside and they are a part of the coccyx, the tail is categorized as typeIa. Though the tail contains a part of vertebrae, they are not excess vertebra and the number of the vertebrae is in the normal range. This type is caused by the mal-curvature or oversize of the coccyx and is not the residual of the embryonic tail. TypeIb tail contains some bony/cartilaginous elements not relevant to the coccyx. This case is highly associated with lipoma or teratoma, and thus these elements seemed to be ectopically formed by those tumors.
When the tail without any bones is located higher than the natal cleft, it is categorized as typeIIa. Among the four human tail types (Ia, Ib, IIa, IIb), this is the most common and neurosurgeons should be most careful to treat this type. It is the most common (92 out of 195 reported cases) and is highly associated with spinal cord malformation (44/92 cases); it is assumed that this could be caused by neural tube closure defects.
The last typeIIb does not contain bones as well and is located within the para-anal region (lower than the natal cleft). Though it is rare, this type of tail occasionally contains striated muscle fibers inside. But these muscle fibers do not originate from the somite (i.e., not related to the erector spinae muscles) but connect to the anus. TypeIIb is often associated with the rectal/anal anomalies, such as imperforate anus, and is possibly the residual of the anal tubercle.
As described, detailed investigation of the case reports, which is based on the basic knowledge of tail anatomy and development, showed us that the cause and appropriate treatment should be different depending on the type, though the symptoms are similar. In addition, this investigation showed that the established belief about the ŌĆ£human tailŌĆØ for a long period of time is not necessarily correct.
CONCLUSION
Although humans do not have tails, clinicians may still obtain some benefit from understanding the purpose and structure of tails in other mammals. Studying the typical structure, development, and functions of tails improves our knowledge of the world and provides us with perspective on this anatomical feature.
Acknowledgements
I would like to thank Professor Ji Yeoun Lee (Department of Anatomy, Seoul National University College of Medicine) and Professor Chae-Yong Kim (Department of Neurosurgery, Seoul National University College of Medicine) for their invitation to write this review.
This work was supported by JSPS KAKENHI Grant Number JP18K15008.
Fig.┬Ā1.
Anatomy of the tail. A : Caudal vertebrae. B : Basic anatomy of caudal musculatures in primates. 1 : M. abductor caudae medialis, 2 : M. abductor caudae lateralis, 3 : M. extensor caudae lateralis, 4 : M. extensor caudae medialis, 5 : M. iliocaudalis+M. pubocaudalis, 6 : M. ischiocaudalis, 7 : M. flexor caudae longus, 8 : M. flexor caudae brevis. 
Fig.┬Ā2.
Tail reduction process during human evolution. A common ancestor species of cercopithecoids and hominoids (Aegyptopithecus zeuxis) possessed a long tail. However, the common ancestral species of hominoids (Proconsul heseloni and Nacholapithecus kerioi) had lost their tails. Humans must also have lost their tails during this period, but no conclusive fossils have been found. 
Fig.┬Ā3.
Development of the tail. A : Gradient of gene expression during axial extension and termination. B : Somites in a human embryo. Somites are present up to the tip of the tail. NT : neural tube, RA : retinoic acid, CNH : chordoneural hinge, PSM : presomitic mesoderm. 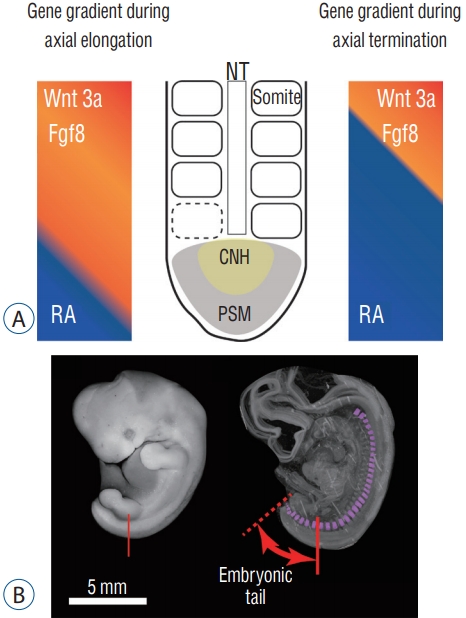
References
1. Altmann SA : A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann N Y Acad Sci 102 : 338-435, 1962   2. Ankel F : Der canalis sacralis als indikator f├╝r die l├żnge der caudalregion der primaten. Folia Primatol (Basel) 3 : 263-276, 1965   3. Ankel F : Vertebral morphology of fossil and extant primates in Tuttle R (ed) : The Functional and Evolutionary Biology of Primates. ed 1. New York : Aldine, 1972, pp223-240
4. Bernstein PL, Smith WJ, Krensky A, Rosene K : Tail positions of Cercopithecus aethiops. Z Tierpsychol 46 : 268-278, 1978  5. Burke AC, Nelson CE, Morgan BA, Tabin C : Hox genes and the evolution of vertebrate axial morphology. Development 121 : 333-346, 1995    6. Dubrulle J, McGrew MJ, Pourqui├® O : FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106 : 219-232, 2001   7. Economides KD, Zeltser L, Capecchi MR : Hoxb13 mutations cause overgrowth of caudal spinal cord and tail vertebrae. Dev Biol 256 : 317-330, 2003   8. Fallon JF, Simandl BK : Evidence of a role for cell death in the disappearance of the embryonic human tail. Am J Anat 152 : 111-129, 1978   9. Favier B, Rijli FM, Fromental-Ramain C, Fraulob V, Chambon P, Doll├® P : Functional cooperation between the non-paralogous genes Hoxa-10 and Hoxd-11 in the developing forelimb and axial skeleton. Development 122 : 449-460, 1996   10. Fleagle JG : Primate Adaptation and Evolution. ed 3. San Diego : Academic Press, 2012
11. Fooden J : Comparative review of fascicularis-group species of macaques (Primates: Macaca). Fieldiana Zoology 2006 : 1-43, 2006  12. Fooden J, Albrecht GH : Tail-length evolution in fascicularis-group macaques (Cercopithecidae: Macaca). Int J Primatol 20 : 431-440, 1999
13. German RZ : The functional morphology of caudal vertebrae in New World monkeys. Am J Phys Anthropol 58 : 453-459, 1982   14. Hall KR : Behaviour and ecology of the wild patas monkey, Erythrocebus patas, in Uganda. J Zool 148 : 15-87, 1966  15. Harrison RG : On the occurrence of tails in man, with a description of the case reported by Dr. Watson. Bull Johns Hopkins Hosp 12 : 96-101, 1901  16. Itani J : Takasakiyama no saru (Japanese monkeys in Takasakiyama) in Imanishi K (ed) : Nihon-Dobutsuki. Tokyo : Kobunsha, 1954
17. Larson SG, Stern JT Jr : Maintenance of above-branch balance during primate arboreal quadrupedalism: coordinated use of forearm rotators and tail motion. Am J Phys Anthropol 129 : 71-81, 2006   18. Maroto M, Bone RA, Dale JK : Somitogenesis. Development 139 : 2453-2456, 2012    19. Nakatsukasa M, Tsujikawa H, Shimizu D, Takano T, Kunimatsu Y, Nakano Y, et al : Definitive evidence for tail loss in Nacholapithecus, an East African Miocene hominoid. J Hum Evol 45 : 179-186, 2003   20. Nakatsukasa M, Ward CV, Walker A, Teaford MF, Kunimatsu Y, Ogihara N : Tail loss in Proconsul heseloni. J Hum Evol 46 : 777-784, 2004   21. Ojha PR : Tail carriage and dominance in the rhesus monkey, Macaca mulatta. Mammalia 38 : 163-170, 1974  22. Organ JM : Structure and function of platyrrhine caudal vertebrae. Anat Rec (Hoboken) 293 : 730-745, 2010   23. Roonwal ML, Tak PC : A field study of subspecific variation in tail form and carriage in the rhesus macaque, Macaca mulatta (Primates). South Asia Bull Zool Surv India 4 : 95-101, 1981
24. Saga Y : The mechanism of somite formation in mice. Curr Opin Genet Dev 22 : 331-338, 2012   25. Sawada A, Shinya M, Jiang YJ, Kawakami A, Kuroiwa A, Takeda H : Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development 128 : 4873-4880, 2001   26. Shapiro LJ : Functional morphology of the vertebral column in primates in Gebo D (ed) : Postcranial Adaptation in Nonhuman Primates. DeKalb : Northern Illinois University Press, 1993, pp121-149
27. Shapiro LJ : Functional morphology of indrid lumbar vertebrae. Am J Phys Anthropol 98 : 323-342, 1995   28. Tojima S : Tail length estimation from sacrocaudal skeletal morphology in catarrhines. Anthropol Sci 121 : 13-24, 2013  29. Tojima S : Variation of the number of proximal caudal vertebrae with tail reduction in old world monkeys. Primates 55 : 509-514, 2014   30. Tojima S : Comparative anatomy of caudal musculature attachments in catarrhines with different tail length. Primate Res 31 : 129-135, 2015  31. Tojima S, Makishima H, Takakuwa T, Yamada S : Tail reduction process during human embryonic development. J Anat 232 : 806-811, 2018    32. Tojima S, Yamada S : Classification of the ŌĆ£human tailŌĆØ: correlation between position, associated anomalies, and causes. Clin Anat 33 : 929-942, 2020   33. Wada N, Nakata A, Koga T, Tokuriki M : Anatomical structure and action of the tail muscles in the cat. J Vet Med Sci 56 : 1107-1112, 1994   34. Ward CV, Walker A, Teaford MF : Proconsul did not have a tail. J Hum Evol 21 : 215-220, 1991  35. Wilson DR : Tail reduction in Macaca in Tuttle R (ed) : The Functional and Evolutionary Biology of Primates. ed 1. New York : Aldine, 1972, pp241-261
36. Young T, Rowland JE, van de Ven C, Bialecka M, Novoa A, Carapuco M, et al : Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell 17 : 516-526, 2009  
|
|



















