Kim, Kim, Kim, Kim, and Yang: Perfusion-Weighted MRI Parameters for Prediction of Early Progressive Infarction in Middle Cerebral Artery Occlusion
Abstract
Objective
Early progressive infarction (EPI) is frequently observed and related to poor functional outcome in patients with middle cerebral artery (MCA) infarction caused by MCA occlusion. We evaluated the perfusion parameters of magnetic resonance imaging (MRI) as a predictor of EPI.
Methods
We retrospectively analyzed patients with acute MCA territory infarction caused by MCA occlusion. EPI was defined as a National Institutes of Health Stroke Scale increment ≥2 points during 24 hours despite receiving standard treatment. Regional parameter ratios, such as cerebral blood flow and volume (rCBV) ratio (ipsilateral value/contralateral value) on perfusion MRI were analyzed to investigate the association with EPI.
Results
Sixty-four patients were enrolled in total. EPI was present in 18 (28%) subjects and all EPI occurred within 3 days after hospitalization. Diabetes mellitus, rCBV ratio and regional time to peak (rTTP) ratio showed statically significant differences in both groups. Multi-variate analysis indicated that history of diabetes mellitus [odds ratio (OR), 6.13; 95% confidence interval (CI), 1.55-24.24] and a low rCBV ratio (rCBV, <0.85; OR, 6.57; 95% CI, 1.4-30.27) was significantly correlated with EPI.
Conclusion
The incidence of EPI is considerable in patients with acute MCA territory infarction caused by MCA occlusion. We suggest that rCBV ratio is a useful neuro-imaging parameter to predict EPI.
Key Words: Acute ischemic stroke · Cerebral blood volume · Hemodynamic failure · Magnetic resonance imaging · Middle cerebral artery occlusion.
INTRODUCTION
Neurological deterioration following stroke is frequently associated with an increased risk of functional disability. Generally, the majority of patients with acute ischemic stroke (AIS) tends to improve about 2-3 days after symptom onset. However, 5% to 40% patients have a chance of neurologic deterioration, so-called early neurological deterioration (END), regardless of the definition used END 27). Major causes of END include symptomatic intracranial hemorrhage, malignant edema, seizure, recurrent or early progression infarction (EPI) stroke and END without a clear pathological mechanism. Thrombus migration from the occlusion site or through hemodynamic instability is reported to be the cause of recurrences or EPI in patients with middle cerebral artery (MCA) occlusion. Although perfusion abnormalities are likely to play a critical role in EPI in acute stroke, there is still uncertainty whether EPI is related to perfusion deficit 1). Although widely used in clinical practice, time-domain perfusion metrics, such as the time to peak (TTP) or the mean transit time (MTT), do not indicate the actual status of collateral flow because of indirect measurement of brain perfusion. Furthermore, the extent of true hemodynamic compromise may be over-estimated. In contrast, regional cerebral blood volume (rCBV) on perfusion magnetic resonance imaging (MRI) reveals that where collateral flow is insufficient to sustain tissue viability and represent "tissue at risk" after an acute ischemic stroke 4,1418). However, there are few report on the relationship between EPI and this perfusion MRI parameter in patients with occluded MCA ischemic stroke who have missed the therapeutic window for intravenous tissue plasminogen activator (t-PA) or intra-arterial thrombolysis. Therefore, in this study, we evaluated useful MRI perfusion parameters to identify the useful predictor of EPI in patients with mild neurologic symptoms but who subsequently developed an acute, large cerebral artery occlusion within a few days of admission.
MATERIALS AND METHODS
We retrospectively collected data from all ischemic stroke patients admitted to our hospital between January 2013 and December 2014. The inclusion criteria for this study were as follows : 1) suspicion of an acute hemispheric infarction involving the MCA territory and confirmed MCA infarction by MR diffusion weighted image (DWI); 2) confirmation of acute occlusion of the MCA main trunk with or without internal carotid artery (ICA) occlusion; 3) hospital arrival between 6 and 24 hours after symptom onset; and 4) diffusion/perfusion mismatch in the MCA territory. Exclusion criteria were as follows : 1) lacunar, posterior circulation or unclassified infarction; 2) intravenous t-PA administration or mechanical thromboectomy procedure for any reason; 3) infarct volume more than 50% in MCA territory at the initial evaluation; 4) severe stroke [National Institutes of Health Stroke Scale (NIHSS) score ≥16]; 5) concurrent with other vascular territory infarctions; 6) patients who failed to complete a MRI within 12 hours after admission; 7) comorbidities that could influence neurological status such as seizure or acute respiratory distress syndrome; 8) cerebral edema or hemorrhagic conversion. All patients were admitted to the stroke unit of the stroke center and treated according to standard protocols. Demographic characteristics, vascular risk factors, baseline laboratory results on the day of admission (blood cell counts, glucose, and C-reactive protein) were obtained from the electric medical record (EMR) database. The severity of the patients' neurological deficits was evaluated according to the NIHSS score immediately after hospital arrival. In the event of neurological deterioration or deterioration of consciousness, a consecutive MR diffusion study was conducted.
Patients were divided into two groups. The EPI group was classified as those individuals experiencing neurological deterioration (NIHSS score increased by more than 2 points during 24 hours) 7,17,31). In addition, MR diffusion images showed an aggravated infarction compared with the initial image. The other individuals who had no change were classified as the static (non-EPI) group. MR perfusion was performed on a 3.0-T Achieva scanner (Philips Medical Systems, Best, The Netherlands). The perfusion imaging data were transferred to a workstation for data processing. CBV, cerebral blood flow (CBF), and MTT maps were generated using the processing software (the Extended MR WorkSpace). A signal-time curve was obtained by capturing the first pass of gadolinium. From this curve, values for CBV and MTT were generated. CBF was obtained using the formula : CBF=CBV/MTT. A TTP map was generated by calculating the difference of the arrival time of the contrast material, and the timing of the maximum contrast agent concentration following its injection. The regions of interest (ROI) were placed in the affected MCA distribution at the supraganglionic level. The mirrored positions to ROI in the normal contralateral hemisphere were used as control ROI. Both ROI and the control ROI (in the contralateral regions) were determined manually. Dynamic susceptibility contrast perfusion MRI was unable to provide absolute values. Therefore, the relative [regional cerebral blood flow (rCBF), rCBV, regional mean transit time (rMTT), regional time to peak (rTTP)] ratios were calculated as the ratio of the affected ROI values to control ROI values (ipsilateral value/contralateral value) ( Fig. 1). An rCBV ratio of 1.0 indicated that normal blood flow was nearly maintained in the ischemic lesion. However, an rCBV ratio <1.0 indicated that CBV in the lesion was reduced compared with the normal contralateral value.
Statistical analysis
Patients were divided into the EPI and non-EPI groups. Categorical variables were expressed as percentages and continuous variables as the mean±standard deviation when the data were normally distributed. Categorical variables were compared using the chi-squared test or Fisher's exact probability test and the medians were compared using the Student's t-test or the Wilcoxon rank sum test. Logistic regression was used to compute unadjusted and multivariable-adjusted odds ratios (OR) for the dichotomous outcomes of the EPI or non-EPI groups. ORs are presented with the 95% confidence intervals (CI). The threshold for statistical significance was set at p<0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences software, version 18 (SPSS, Chicago, IL, USA).
RESULTS
Within the study period, 172 patients were diagnosed with acute MCA stroke combined with MCA occlusion. Of these, sixty four patients satisfied both the inclusion and exclusion criteria and eighteen patient (28%) out of them developed EPI, whereas 46 (72%) did not (non-EPI group). All EPI occurred within 3 days of admission. Ten patients from the EPI group (55%, 10/18) had urgent EC-IC bypass surgery [superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis] : 90% (9/10) had a good outcome (3 months modified rankin scale (mRS) <3). However, the other eight patients (non-surgical EPI patients) had poor outcomes (3 months mRS ≥3).
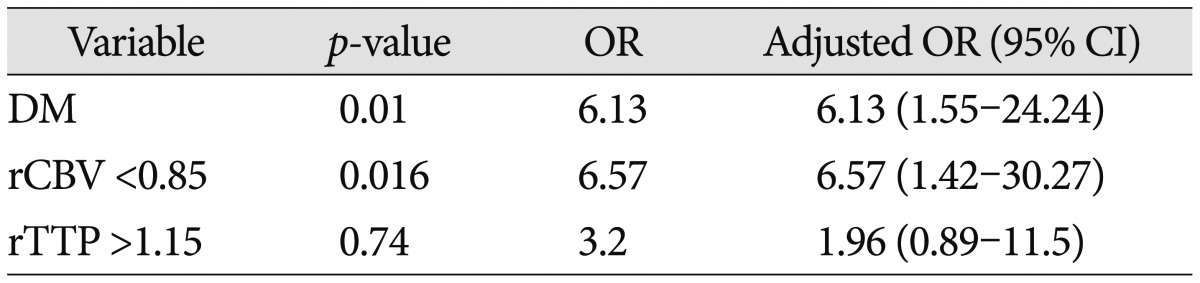
The patients' ages and sex were similar in the EPI and non-EPI groups. Past medical histories such as hypertension, atrial fibrillation, hyperlipidemia, and smoking were similar in both groups except for diabetes mellitus (DM) (55% vs. 21% in the EPI and non-EPI groups respectively, p=0.009). Furthermore, NIHSS scores at admission were not statistically significant ( Table 1). With regard to radiological findings at the time of admission, occlusion of both MCA and ICA were more frequently observed in the EPI group compared to the non-EPI group, but the difference between the groups was not statistically significant (34% vs. 27%, p=0.154). The rCBF and rMTT ratio were lower in the EPI group but the difference was not statistically significant (rCBF : 0.89 vs. 0.73, p=0.069 and rMTT : 1.32 vs. 1.22, p=0.075). In contrast, the EPI group displayed significantly decreasing rCBV and an increasing rTTP ratio (rCBV : 1.13 vs. 0.95, p=0.045; rTTP : 1.11 vs. 1.2, p=0.019). The rCBF, rCBV, rMTT, and rTTP ratio was dichotomized to 0.7, 0.85, 1.15 and 1.15 thresholds, respectively, according to other perfusion studies 3,2629). Furthermore, NIHSS was dichotomized to 8 (NIHSS ≥8) 33). For univariate analyses, the following factors were found to be significantly different between the non-EPI and EPI group : DM, rCBF <0.7, rCBV <0.85, and rTTP >1.15 ( Table 2). From the multivariate analysis, two parameters were identified as valuable predictive factors for EPI : DM (OR, 6.1; 95% CI, 1.55-24.2; p=0.01) and rCBV <0.85 (OR, 6.6; 95% CI, 1.4-30.3; p=0.016) ( Table 3).
DISCUSSION
The present study demonstrates that underlying DM and a decreasing rCBV ratio on perfusion MRI is a potential predictor of EPI. In our series, EPI, which was defined as those individuals experiencing neurological deterioration (NIHSS score increased by more than 2 points during 24 hours), developed in 28% patients with MCA infarction caused by MCA occlusion within 3 days. This finding is similar to the European Study Progressing Stroke Study, which showed that episodes of early deterioration occurred in 33% of patients 2). Other studies corroborate that EPI is common, occurring in 26-43% of patients, and about half of all cases occur within 1 day of hospital admission 5,1230). These variations in the prevalence may be due to patient characteristics, stroke type and the use of different diagnostic criteria in different studies. Our study did not show any statistically significant association between EPI and the patient's age, gender, or other previous medical history other than DM. It is well known that DM increases the risk of stroke several-fold. Moreover, the combination of stroke and DM is associated with a poorer stroke-related outcome, high disability, and stroke recurrence 11,24). However, the exact mechanism of how DM affects EPI is less clear. Several studies have revealed the contribution of chronic hyperglycemia to underlying coagulation activation 20,32). Furthermore, hyperglycemia is an important determinant of infarct volume expansion, even in patients with good collateral circulation or early recanalization 15,28). In addition, hyperglycemia induces progressive cerebrovascular changes during ischemia and affects hemodynamic recovery after reperfusion 13). A recent meta-analysis emphasized that history of DM was associated with poor clinical outcome in AIS patients, even when treated with IV or IA thrombolysis 6). A possible explanation for this is that the ischemic brain tissue is more vulnerable in patients with diabetes mellitus because of specifically-affected structure and function of the vascular bed 8). Furthermore, diabetic microangiopathy may alter cerebral autoregulation and affect the collateral circulation, making affected patients more sensitive to hypoperfusion. This would consequently increase the risk of deepening and extending the infarction 10). When MCA occludes, there are broadly two territories of decreased perfusion : an area of ischemia and a region of oligemia. The area of oligemia is characterized by a moderate reduction in flow, yet is sufficient to maintain ionic homeostasis. This lesion is likely to be exacerbated by infarction but is an essential therapeutic target. In this aspect, recent developments in diffusion and perfusion MRI have provided useful information about the ischemic penumbra, the tissue at risk. After acute ischemic stroke, this territory can be salvaged by recanalization or reperfusion. In the early stages of ischemia, decreased cerebral perfusion pressure produces vasodilatation and an increase in CBV. With further reduction of cerebral perfusion pressure, compensatory vasodilatation goes from its maximum level to vascular collapse. In some studies, decreased CBV areas are well correlated with the final infarction volume, because infarct growth is related to the extent of collateral circulation 16,25). CBV on perfusion MRI represents the extent of collateral circulation and the extent of vasodilatation 19,21). Therefore, a lower rCBV ratio on MR perfusion weighted image (PWI) may indicate an insufficient collateral flow. Our study also revealed that the rCBV ratio was decreased compared with the normal contralateral side (rCBV <0.85; OR, 6.6; 95% CI, 1.4-30.3; p=0.016) and was associated with EPI. In this series, TTP is also associated with EPI but this was not statistically significant (rTTP >0.15; OR, 1.96; 95% CI, 0.9-311.5; p=0.74). Generally time-based parameters such as MTT or TTP have been considered the most reliable methods for ischemic stroke. However, these have several weak points. It is an indirect measurement of brain perfusion and easily contaminated by the severe arterial occlusive states such as chronic occlusion, which leads to an overestimation of the true extent of hemodynamic compromise in acute ischemic stroke 22,34). Our study also support that rCBV map is superior to time-based perfusion maps (TTP) for predicting the tissue at risk of EPI. Although the rTTP ratio increases, and rCBV ratio >1, the risk of EPI may be low because of early collateral build up. In contrast, the rCBV ratio <0.85 and increasing rTTP ratio represent poor collateral circulation and are associated with high risk of EPI ( Fig. 2). It is important to provide appropriate treatment for patients with EPI despite intention to provide full medical treatment, as well as prediction of EPI. Extracranial-intracranial (EC-IC) bypass surgery is considered beneficial in selective patients with symptomatic ICA occlusion and concomitant hemodynamic instability, despite full medical therapy. However, there are somewhat controversy with use of STA-MCA anastomosis as an urgent treatment. Furthermore, there is no current consensus on the optimal timing of bypass surgery, particularly in patients with progressive infarction accompanied by hemodynamic instability, which does not respond to the best available treatment. A small case series recently reported that urgent STA-MCA bypass stabilized the progression of ischemia and/or reversed the symptoms in majority of the cases. In addition, there were no surgical complications or hemorrhage 9,1823). Thus, it may be possible to prevent an ongoing ischemic event thereby stopping and/or reversing neurological symptoms. This study has two potential limitations. Firstly, this study was conducted with a retrospective design and was not a randomized trial. Secondly, this study included a small number of patients at a single institution, the validity of our results cannot be absolutely confirmed. Nevertheless, the results of this study could raise the clinical attention to predict EPI using perfusion MRI. A multi-center study including multimodal radiologic imaging with a larger sample size is required to confirm our results.
CONCLUSION
Our study proved that the rCBV ratio assessed by perfusion MRI can be used to predict EPI in patients with acute ischemic stroke combined with MCA occlusion. It may useful for early prediction of EPI development as well as the ability to select patients in need of urgent EC-IC bypass surgery, such as STA-MCA. Further large and prospective studies are necessary to establish correlations with clinical findings and multimodal neuro-imagings.
References
1. Alawneh JA, Moustafa RR, Baron JC : Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke 2009, 40 : e443-e450,   2. Birschel P, Ellul J, Barer D : Progressing stroke : towards an internationally agreed definition. Cerebrovasc Dis 2004, 17 : 242-252,   3. Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al : Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011, 42 : 3435-3440,   4. Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al : Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013, 33 : 1168-1172,    5. Dávalos A, Cendra E, Teruel J, Martinez M, Genís D : Deteriorating ischemic stroke : risk factors and prognosis. Neurology 1990, 40 : 1865-1869,   6. Desilles JP, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, et al : Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis : a registry and systematic review. Stroke 2013, 44 : 1915-1923,   7. Eusebi P : Diagnostic accuracy measures. Cerebrovasc Dis 2013, 36 : 267-272,   8. Hjalmarsson C, Manhem K, Bokemark L, Andersson B : The role of prestroke glycemic control on severity and outcome of acute ischemic stroke. Stroke Res Treat 2014, 2014 : 694569,     9. Horiuchi T, Nitta J, Ishizaka S, Kanaya K, Yanagawa T, Hongo K : Emergency EC-IC bypass for symptomatic atherosclerotic ischemic stroke. Neurosurg Rev 2013, 36 : 559-564; discussion 564-565,   10. Hou Q, Zuo Z, Michel P, Zhang Y, Eskandari A, Man F, et al : Influence of chronic hyperglycemia on cerebral microvascular remodeling : an in vivo study using perfusion computed tomography in acute ischemic stroke patients. Stroke 2013, 44 : 3557-3560,   11. Jia Q, Zhao X, Wang C, Wang Y, Yan Y, Li H, et al : Diabetes and poor outcomes within 6 months after acute ischemic stroke : the China National Stroke Registry. Stroke 2011, 42 : 2758-2762,   12. Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS : Effect of blood pressure and diabetes on stroke in progression. Lancet 1994, 344 : 156-159,   13. Kawai N, Keep RF, Betz AL : Hyperglycemia and the vascular effects of cerebral ischemia. Acta Neurochir Suppl 1997, 70 : 27-29,   14. Khatri R, Rodriguez GJ, Suri MF, Vazquez G, Ezzeddine MA : Lepto meningeal collateral response and computed tomographic perfusion mismatch in acute middle cerebral artery occlusion. J Vasc Interv Neurol 2011, 4 : 1-4,
15. Kimura K, Sakamoto Y, Iguchi Y, Shibazaki K, Aoki J, Sakai K, et al : Admission hyperglycemia and serial infarct volume after t-PA therapy in patients with and without early recanalization. J Neurol Sci 2011, 307 : 55-59,   16. Kluytmans M, van Everdingen KJ, Kappelle LJ, Ramos LM, Viergever MA, van der : Prognostic value of perfusion- and diffusion-weighted MR imaging in first 3 days of stroke. Eur Radiol 2000, 10 : 1434-1441,   17. Kwan J, Hand P : Early neurological deterioration in acute stroke : clinical characteristics and impact on outcome. QJM 006, 99 : 625-633,  18. Lee SB, Huh PW, Kim DS, Yoo DS, Lee TG, Cho KS : Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg 2013, 115 : 1238-1244,   19. Lee SY, Cha JK, Kang MJ : Regional cerebral blood volume ratio on perfusion MRI on the growth of infarct size in acute ischemic stroke. Eur Neurol 2009, 62 : 281-286,   20. Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB : Hyperglycemia : a prothrombotic factor? J Thromb Haemost 2010, 8 : 1663-1669,   21. Liu Y, Karonen JO, Vanninen RL, Nuutinen J, Koskela A, Soimakallio S, et al : Acute ischemic stroke : predictive value of 2D phase-contrast MR angiography--serial study with combined diffusion and perfusion MR imaging. Radiology 2004, 231 : 517-527,   22. Neumann-Haefelin T, Wittsack HJ, Fink GR, Wenserski F, Li TQ, Seitz RJ, et al : Diffusion- and perfusion-weighted MRI : influence of severe carotid artery stenosis on the DWI/PWI mismatch in acute stroke. Stroke 2000, 31 : 1311-1317,   23. Nussbaum ES, Janjua TM, Defillo A, Lowary JL, Nussbaum LA : Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. J Neurosurg 2010, 112 : 666-673,   24. Reeves MJ, Vaidya RS, Fonarow GC, Liang L, Smith EE, Matulonis R, et al : Quality of care and outcomes in patients with diabetes hospitalized with ischemic stroke : findings from Get With the Guidelines-Stroke. Stroke 2010, 41 : e409-e417,   25. Schaefer PW, Hunter GJ, He J, Hamberg LM, Sorensen AG, Schwamm LH, et al : Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. AJNR Am J Neuroradiol 2002, 23 : 1785-1794,   26. Schaefer PW, Souza L, Kamalian S, Hirsch JA, Yoo AJ, Kamalian S, et al : Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke 2015, 46 : 419-424,   27. Seners P, Turc G, Oppenheim C, Baron JC : Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke : a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 2015, 86 : 87-94,   28. Shimoyama T, Shibazaki K, Kimura K, Uemura J, Shiromoto T, Watanabe M, et al : Admission hyperglycemia causes infarct volume expansion in patients with ICA or MCA occlusion : association of collateral grade on conventional angiography. Eur J Neurol 2013, 20 : 109-116,   29. Srinivasan A, Goyal M, Al Azri F, Lum C : State-of-the-art imaging of acute stroke. Radiographics 2006, 26( Suppl 1):S75-S95,   30. Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, Argentino C, et al : Progressing neurological deficit secondary to acute ischemic stroke. A study on predictability, pathogenesis, and prognosis. Arch Neurol 1995, 52 : 670-675,   31. Toyoda K, Fujimoto S, Kamouchi M, Iida M, Okada Y : Acute blood pressure levels and neurological deterioration in different subtypes of ischemic stroke. Stroke 2009, 40 : 2585-2588,   32. Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G : Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 2006, 55 : 202-208,   33. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al : Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) : an observational study. Lancet 2007, 369 : 275-282,   34. Yamada K, Wu O, Gonzalez RG, Bakker D, Østergaard L, Copen WA, et al : Magnetic resonance perfusion-weighted imaging of acute cerebral infarction : effect of the calculation methods and underlying vasculopathy. Stroke 2002, 33 : 87-94,  
Fig. 1
A 68 year-old female patient visited the emergency room, 8 hours after symptom onset. Initial NIHSS was 5. A and B : MR images revealed occlusion in the left, proximal middle cerebral artery and multiple subcortical infarctions on the frontal. C : Region of interest (yellow line) is highlighted in the affected cerebral hemisphere within the middle cerebral artery territory by manual segmentation and then mirrored to the contralateral cerebral hemisphere for assessment of perfusion ratio. rCBV ratio was decreased and rTTP ratio was increased. D : Fourteen hours after hospitalization, neurologic deterioration had occurred and follow-up MR image revealed extended infarction. E : We performed urgent superficial-middle cerebral artery bypass surgery and brain CT angiogram revealed transcranial passage of STA parietal branch (yellow arrow). F : Patients improved gradually and finally, the presence of neurological deficit was assessed using modified Rankin scale 2, three months later. NIHSS: National Institutes of Health Stroke Scale, rCBV : regional cerebral blood volume, rTTP : regional time to peak, STA : superficial temporal artery.
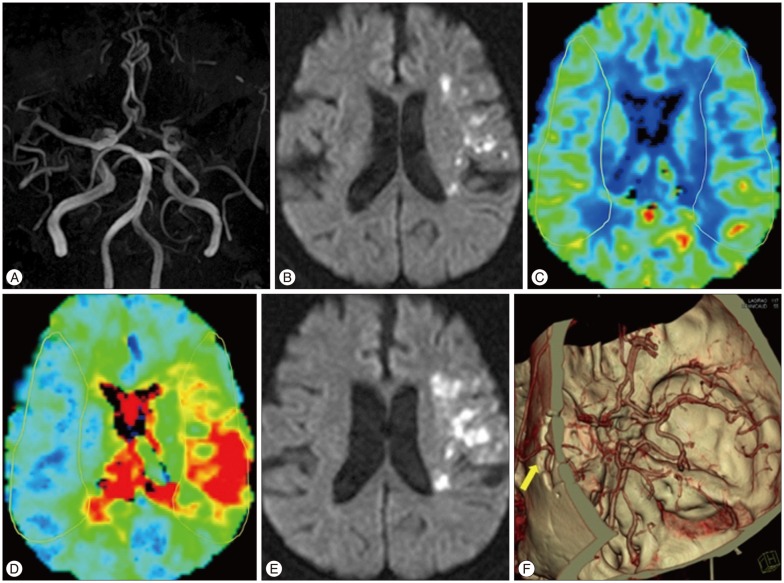
Fig. 2
The correlation between rCBV ratio and the rTTP ratio according to early progressive infarction. Red circle represents EPI and blue represents the non-EPI group. Area above the blue dotted line may represent early collateral build up (although the rTTP ratio increases, rCBV ratio >1). In contrast, the gray area has a high risk of EPI because of poor collateral circulation.
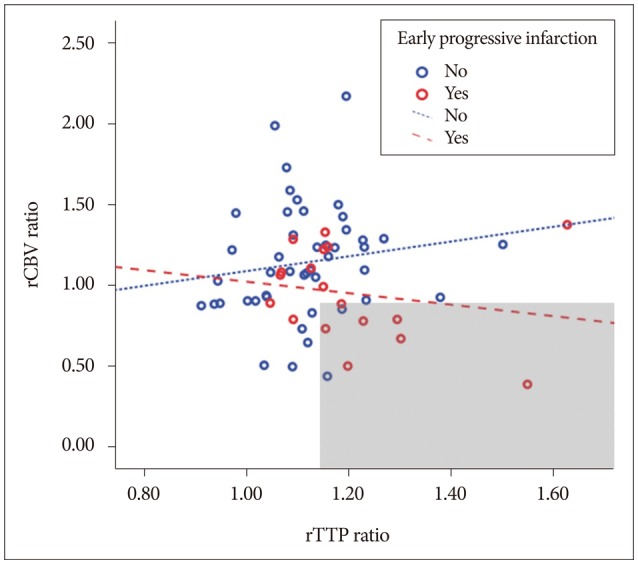
Table 1
Comparisons of the baseline features and MR perfusion parameter between the EPI and non-EPI groups
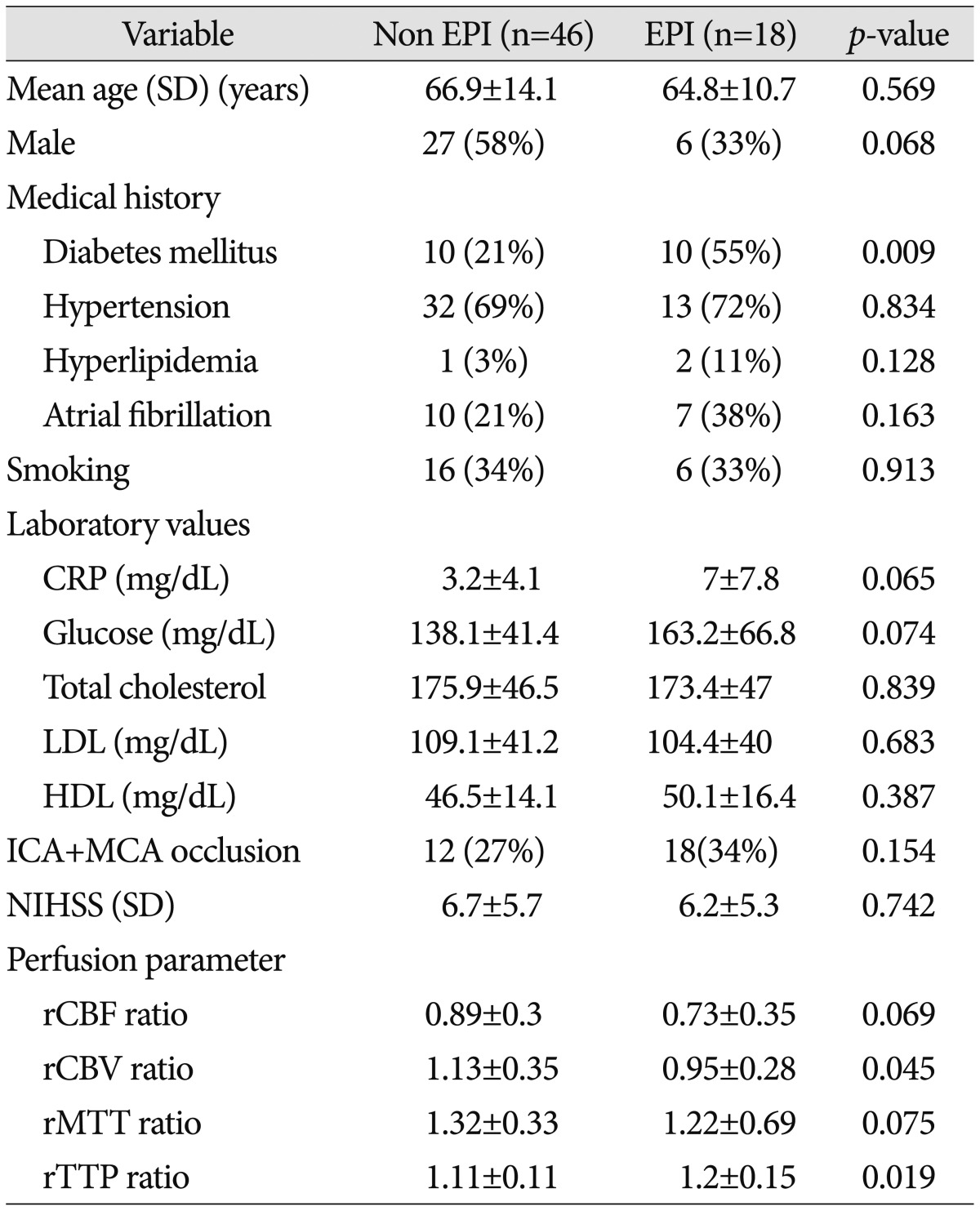
Table 2
Sipmle logistic regression analysis for occurrence of early progressive infarction
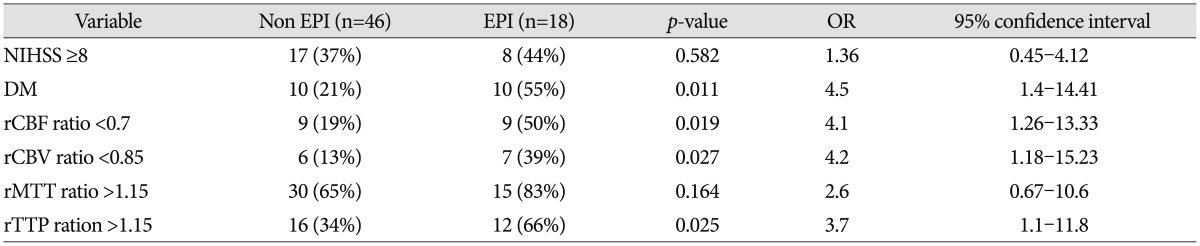
Table 3
Risk factors for early progressive infarction using multivariate logistic regression
|
|




















