Kim, Ko, Kang, Seo, Park, Sheen, Park, Kang, Kim, Oh, Hong, Yu, Heo, Kwon, Bae, Lee, Yoon, Park, and Rha: Clinical Practice Guidelines for the Medical and Surgical Management of Primary Intracerebral Hemorrhage in Korea
Abstract
The purpose of this clinical practice guideline (CPG) is to provide current and comprehensive recommendations for the medical and surgical management of primary intracerebral hemorrhage (ICH). Since the release of the first Korean CPGs for stroke, evidence has been accumulated in the management of ICH, such as intracranial pressure control and minimally invasive surgery, and it needs to be reflected in the updated version. The Quality Control Committee at the Korean Society of cerebrovascular Surgeons and the Writing Group at the Clinical Research Center for Stroke (CRCS) systematically reviewed relevant literature and major published guidelines between June 2007 and June 2013. Based on the published evidence, recommendations were synthesized, and the level of evidence and the grade of the recommendation were determined using the methods adapted from CRCS. A draft guideline was scrutinized by expert peer reviewers and also discussed at an expert consensus meeting until final agreement was achieved. CPGs based on scientific evidence are presented for the medical and surgical management of patients presenting with primary ICH. This CPG describes the current pertinent recommendations and suggests Korean recommendations for the medical and surgical management of a patient with primary ICH.
Key Words: Clinical · Guideline · Treatment · Spontaneous · Intracerebral hemorrhage.
INTRODUCTION
The ideal treatment of intracerebral hemorrhage (ICH) is to minimize on-going neurologic damage including limiting hematoma expansion and early management of neurologic complications. If surgically indicated, removal of the hematoma may be considered while minimizing direct brain damage associated with the surgery itself. In some cases, conservative treatment may have priority over surgery, depending on the location and the size of the hemorrhage. Moreover, many different surgical methods, either tried or being developed, are available. Therefore, clear guidelines are needed in this regard.
The Korean Society of Cerebrovascular Surgeons (KSCVS) and the Clinical Research Center for Stroke (CRCS) decided to issue a Korean version of the guidelines for the medical and surgical management of primary ICH as a framework for the treatment decision and as a basis for future studies, following the 'Guideline Development Manual' of CRCS. The Quality Control Committee (QCC) at the KSCVS and the Writing Group (WG) at the CRCS systematically reviewed relevant literature and major published guidelines between June 2007 and June 2013 and took a developmental strategy of adaptation rather than de novo methods. Based on the interpretation of the published evidence, recommendations were made, and the level of evidence and the grade of the recommendation were determined using the methods adapted from those of the CRCS. Though guidelines are also needed on ICH secondary to cerebral aneurysm or cerebral arteriovenous malformation which requires surgical removal, only primary ICH is covered in the present clinical practice guideline (CPG). The latest evidence is reflected in this CPG in a timely manner to help physicians and their patients make a decision in clinical practice. Therefore, revision of this CPG by newly released evidence from ongoing trials will be achieved, and subjects that are not covered in this CPG due to insufficient scientific evidence are believed to be major research topics in the future. Lack of domestic references also remains an unsolved issue. The authors definitely emphasize and make it very clear that the ultimate discretion is always with the physician in charge who has a broad perspective of the various factors in play in each patient. The present CPG, therefore, is neither to limit the practice of medicine by any healthcare professional, nor to provide reference for insurance claim evaluation. Furthermore, it should never serve as a basis for legal judgment for a medical care which was provided under a specific clinical situation.
MEDICAL MANAGEMENT OF PRIMARY ICH
Medical management of ICH can be classified into three parts : 1) blood pressure (BP) control to possibly limit hematoma expansion, 2) managing intracranial pressure (ICP) crisis, and 3) management of seizures. Here, we will review the recent advances in these issues.
BP management
The following guidelines are recommended for BP control in acute ICH [level of evidence (LOE) : III, Grade of Recommendation (GOR) : B] ( Table 1). 1) For systolic BP (SBP) >200 mm Hg or mean arterial pressure (MAP) >150 mm Hg, measure BP every 5 minutes, and lower BP aggressively with intravenous antihypertensives.
2) For SBP >180 mm Hg or MAP >130 mm Hg with (suspected) ICP elevation, maintain cerebral perfusion pressure (CPP) at 60-80 mm Hg, and lower BP with intravenous antihypertensives (bolus or infusion).
3) For SBP >180 mm Hg or MAP >130 mm Hg without evidence of ICP elevation, clinically evaluate the patient every 15 minutes, and control BP with intravenous antihypertensives (bolus or infusion) to maintain a MAP of 110 mm Hg or SBP/diastolic BP (DBP) of 160/90 mm Hg.
Recommendations from foreign guidelines
American Heart Association/American Stroke Association (AHA/ASA, USA) 52)
1) Until ongoing clinical trials of BP intervention for ICH are completed, physicians must manage BP based on the present incomplete efficacy evidence. Current suggested recommendations for target BPs in various situations are listed below and may be considered (Class IIb; LOE C)
i. If SBP is >200 mm Hg or MAP is >150 mm Hg, then consider aggressive reduction of BP with continuous intravenous infusion, with frequent BP monitoring every 5 minutes.
ii. If SBP is >180 mm Hg or MAP is >130 mm Hg and there is the possibility of elevated ICP, then consider monitoring ICP and reducing BP using intermittent or continuous intravenous medications while maintaining a CPP of ≥60 mm Hg.
iii. If SBP is >180 mm Hg or MAP is >130 mm Hg and there is no evidence of elevated ICP, then consider a modest reduction of BP (e.g., a MAP of 110 mm Hg or a target BP of 160/90 mm Hg) using intermittent or continuous intravenous medications to control BP and clinically reexamine the patient every 15 minutes.
2) In patients presenting with a SBP of 150 to 220 mm Hg, acute lowering of SBP to 140 mm Hg is probably safe (Class IIa; LOE B) (new recommendation).
New evidences
The previous recommendations on BP management in patients with acute ICH were mainly based on small observational studies. An anecdotal report showed that an admission SBP >200 mm Hg was associated with hematoma expansion on follow up CT scan. Therefore, a SBP <200 mm Hg was initially recommended as a BP goal 38). However, lowering BP may not always be safe because the majority of patients have a history of hypertension (HT). Considering that the autoregulation curve is shifted toward the higher ranges of BP in patients with HT, there is a safety concern regarding the acute reduction of BP. Therefore, a modest recommendation was made in the previous guidelines for safety reasons. An Initial practice guideline suggested that a SBP <180-185 mm Hg with a DBP <105-110 mm Hg was recommended in patients with a history of HT, while a SBP <160-170 mm Hg with a DBP <95-100 mm Hg was recommended in those without a history of HT 13). Those recommendations were rephrased in terms of MAP, and a MAP < 130 mm Hg was suggested in the 1999 and 2007 American Stroke Association (ASA) guidelines 10,11). After the ASA ICH guideline in 2007, three major clinical trials were published, which focused on the relationship between acute BP reduction and clinical outcome. In 2008, Intensive blood pressure reduction in acute cerebral hemorrhage Trial (IN-TERACT) result came out 3). Included patients had acute spontaneous ICH diagnosed by CT within 6 hours of onset, an elevated SBP (150 to 220 mm Hg), and no definite indication or contraindication to BP control. They were randomly assigned to early intensive BP lowering (a target SBP of 140 mm Hg; n=203) or standard guideline-based BP management (a target SBP of 180 mm Hg; n=201). While the mean proportional hematoma growth after 24 hours was lower in the intensive group compared to the standard treatment group [13.7% vs. 36.3%, absolute difference 22.6%, 95% confidence interval (CI) 0.6-44.5%; p=0.04], intensive BP-lowering did not increase the risks of adverse events, but failed to improve clinical outcomes at 90 days. Although intensive BP reduction may be safe, the absolute difference in hematoma volume between the two groups was only 1.7 mL, which raised the question regarding the effectiveness of intensive BP reduction from a practical standpoint. In addition, the majority of patients (85% of the intensive group, and 95% of the conventional group) were treated with diuretics or vasodilators, which were not commonly used for controlling BP in usual practice. Despite these limitations, intensive BP reduction may be safely tried in patients with acute ICH. Another study reflecting a real practice pattern came out in 2010 5). The Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trial also included patients with acute ICH within 6 hours from symptom onset if they had an elevated SBP ≥170 mm Hg. Patients were randomly allocated into three tiers of SBP goals (170 to 200 mm Hg, n=18 ; 140 to 170 mm Hg, n=20; or 110 to 140 mm Hg, n=22), and SBPs were controlled with intravenous nicardipine infusion. Primary outcomes were treatment feasibility and safety up to 72 hours. Serious adverse events were observed in one subject (5%) in tier two (SBP 140 to 170 mm Hg) and in three subjects (14%) in tier three (SBP 110 to 140 mm Hg). However, the safety stopping rule was not activated in any of the tiers. Therefore, controlling BP with nicardipine may be safe 5). In order to explore its clinical utility, a phase 3 clinical trial is currently underway (ATACH-II) 65). Recently, the result of the INTERACT II, a phase 3 trial, was published 2). A total of 2839 patients with acute ICH within 6 hours from symptom onset were randomized to two BP goals (a target SBP <140 mm Hg within 1 hour vs. SBP <180 mm Hg) if their SBP was between 150 mm Hg and 220 mm Hg. The antihypertensive drugs were chosen at the discretion of the treating physicians. The primary outcome was a combined endpoint of death and major disability (mRS 3 to 6) at 3 months. The intensive treatment group had a tendency for less death or major disability (52.0%) compared to those in the guideline group (55.6%) (odds ratio with intensive treatment, 0.87; 95% CI, 0.75 to 1.01; p=0.06), which failed to reach statistical significance. However, secondary outcome with the ordinal analysis on the mRS showed a significantly lower mRS with intensive treatment (odds ratio for greater disability, 0.87; 95% CI, 0.77 to 1.00; p=0.04). Safety outcome was not different between the two groups. The main mechanism for the improv-ed outcomes with rapid intensive BP reduction is still unclear because there were no significant absolute or relative changes in hematoma growth between the two treatment groups. The hematoma volume difference between the groups was very small (adjusted mean volume, 1.4 mL). The major safety concern regarding acute BP reduction is the potential for aggravation of ischemic injury due to the possible existence of perihematomal penumbra. In order to clarify this issue, a randomized open-label study with blinded evaluation was carried out 14). The included patients were >18 years of age with acute spontaneous ICH within 24 hours after onset, and had a SBP greater than 150 mm Hg. Patients were randomized to an SBP target of <150 mm Hg (n=39) or <180 mm Hg (n=36) and underwent a CT perfusion scan to compare the difference in relative cerebral blood flow (rCBF) between the two treatment groups. There was no relationship between the BP change and perihematomal rCBF in both treatment groups suggesting the safety of acute BP reduction in patients with ICH. Based on these results, rapid BP reduction to a SBP <140 mm Hg in patients with acute ICH is probably safe, and may be beneficial in terms of improving functional outcome.
New recommendations
1. The suggested BP targets in patients with acute ICH are described below (LOE III, GOR B). Drugs that can be used for BP control in spontaneous ICH are shown in Table 2 (revised recommendation). 1) If the SBP is >200 mm Hg or MAP is >150 mm Hg, then consider aggressive BP reduction with a continuous intravenous infusion of drugs, with frequent BP monitoring every 5 minutes.
2) If the SBP is >180 mm Hg or MAP is >130 mm Hg and there is any possibility of an ICP elevation, then consider ICP monitoring and reducing BP using an intermittent or continuous intravenous infusion while maintaining a CPP (MAP-ICP) of 50-70 mm Hg.
3) If the SBP is >180 mm Hg or MAP is >130 mm Hg and there is no evidence of an ICP elevation, then consider a modest reduction of BP using an intermittent or continuous intravenous infusion (MAP of 110 mm Hg for a BP of 160/90 mm Hg) and clinically reexamine the patient every 15 minutes.
2. In patients with acute ICH, when the SBP is measured between 150 and 220 mm Hg, the SBP may be safely lowered to 140 mm Hg within 1 hour (LOE Ib, GOR A) (new Recommendation).
The management of intracranial pressure elevation
ICP elevation is one of the most common reasons for secondary damage in patients with ICH 47). Hematoma expansion, perihematomal edema, or hydrocephalus will further aggravate ICP surges. An ICP crisis will result in cerebral herniation and subsequently a poor neurologic outcome. Therefore, ICP elevation should be properly managed in patients with ICH. After the publication of the first Korean version of the ICH guideline in 2009, no high quality clinical study has been done regarding ICP management in patients with ICH. Meanwhile, the ASA ICH guideline was updated based on the results from traumatic brain injury (TBI) trials. Therefore, the management effect on ICP should be interpreted with great caution in this regard.
1. Treatment and monitoring at the intensive care unit (ICU) is recommended in acute hemorrhage patients because of the need for ICP elevation monitoring, BP control, intubation, and mechanical ventilation (LOE III, GOR B).
2. To control an elevated ICP, a gradual treatment should be considered. The patient's head should be elevated by about 30° to start with. Limited use of analgesics and/or sedatives may be considered in patients with pain and/or unstable conditions (LOE IV, GOR C).
3. A more aggressive ICP control includes the use of mannitol and hypertonic saline solution, CSF drainage via a ventricular catheter, neuromuscular blockade, and hyperventilation. In general, maintaining a CPP ≥70 mm Hg is recommended along with the close monitoring of the ICP and blood pressure (LOE IV, GOR C).
Recommendations from foreign guidelines
1. People with ICH should be monitored by specialists in neurosurgical or stroke care for deterioration in function and referred immediately for brain imaging when necessary [Royal College of Physicians (RCP), UK 2008, LOE consensus].
2. Patients with a Glasgow Coma Scale (GCS) score of ≤8, those with clinical evidence of transtentorial herniation, or those with significant intraventricular hemorrhage (IVH) or hydrocephalus could be considered for ICP monitoring and treatment. A CPP of 50 to 70 mm Hg may be reasonable to maintain depending on the status of cerebral autoregulation (AHA/ASA, USA 2010 : Class IIb; LOE C).
3. Ventricular drainage as a treatment for hydrocephalus is reasonable in patients with a decreased level of consciousness (AHA/ASA, USA 2010 : Class IIa; LOE B).
4. Corticosteroid should not be used for the treatment of primary ICH [Scottish Intercollegiate Guidelines Network (SIGN) 2008 : LOE 1++, GOR B].
5. Intravenous mannitol should not be used routinely for the treatment of raised intracranial pressure in patients with primary ICH (SIGN 2008 : LOE 1+, GOR B).
New evidences
An accurate ICP measurement is the first step in managing patients with ICP elevation. Intraparenchymal monitor may better reflect perihematomal ICPs, representing a more accurate hemodynamic status in the perilesional area which is vulnerable to secondary injury. ICP measured by ventricular catheter (VC) represents global ICP, which is regarded as a gold standard. Given that VC can be used to drain cerebrospinal fluid or intraventricular hemorrhage, ICP measurement via VC is preferred in patients with concomitant IVH. The procedural complication rate is reported to be as high as 3% to 4% including procedural hemorrhage and catheter related infection 24). The frequency and risk factors for ICP crisis in patients with ICH remains to be elucidated. In small randomized studies, an initial ICP, measured at the time of VC insertion, was not directly associated with the IVH volume or parenchymal ICH volume 79). Moreover, the mean daily ICP was not correlated with hemorrhage burden 79). Although the existence of IVH per se does not mean an ICP crisis, the presence of IVH is considered to be associated with poor prognostic factors, probably mediated by hydrocephalus. In the International Surgical Trial in Intracerebral Haemorrhage (STICH) trial, 377 (42%) out of 902 patients had IVH, and a total of 208 (23% among the study patients; 55% of the patients with IVH) developed hydrocephalus. Favorable functional outcomes at 6 months occurred in 15% and 31% of the patients with and without IVH ( p<0.00001), respectively, suggesting an association between the presence of IVH and functional outcome 44). Moreover, if hydrocephalus is combined in patients with IVH, the percentage of favorable outcomes drops from 20% to 12% ( p<0.031). Therefore, the presence of hydrocephalus is considered as an important prognostic factor 7). In addition, the presence of IVH is accepted as an important prognostic factor to predict mortality at 1 month and functional recovery at 12 months, which is reflected in the ICH score 17,27,28). Therefore, ICP measurement is necessary, especially in patients who have IVH or hydrocephalus, and the insertion of VC may be considered when their mental status deteriorates as hydrocephalus develops. The indications of ICP monitoring is still unclear, but it may be tried in ICH patients with significant severity (GCS score of 3 to 8), based on the TBI guideline in 2008 8,57). ICP monitoring is needed in patients with huge supratentorial lesions because those patients are at high risk for transtentorial herniation with secondary brainstem compression 4,64). Therefore, patients with clinical signs of transtentorial herniation may be a good candidate for ICP monitoring. If ICP can be measured, CPP can be calculated from the difference between MAP and ICP. It is still debatable regarding which ranges of CPP should be pursued in patients with ICH. The AHA/ASA ICH guideline in 2007 suggested that CPP should be maintained greater than 70 mm Hg, and the target level was lowered to a CPP of 50 to 70 mm Hg in 2010 52). Those recommendations are adapted from the 2007 TBI guideline from the brain trauma foundation. It recommended that a CPP lower than 50 mm Hg should be avoided (Level III) and not be maintained greater than 70 mm Hg (Level II) 9). The lower limit of CPP (>50 mm Hg) is based on a small study. In patients with severe TBI with a depressed CPP level around 32 mm Hg, if the CPP was elevated up to 68 mm Hg with the proper use of pressors, the partial brain tissue oxygen pressure (PbtO2) improved. Based on the fact that the PbtO2 level is associated with a poor functional outcome, a low CPP should be avoided as well 39). Moreover, in a small observational study using microdialysis, when the CPP dropped below 50 mm Hg, the lactate/pyruvate ratio surged, reflecting a metabolic crisis and anaerobic shift of brain glucose metabolism 61). Taken together, a CPP less than 50 mm Hg should be avoided 9). The upper range of the CPP at 70 mm Hg is also based on studies in patients with severe TBI 66). Severe TBI patients were randomized to either a CPP >70 mm Hg or a CPP 50-70 mm Hg, and the frequency of sustained jugular venous desaturation (JvSO2) <50% more than 10 minutes, was less in patients with higher CPP goals (CPP >70 mm Hg), while the functional outcome was not different. However, the development of acute respiratory difficulty syndrome was 5 times more frequent in the higher CPP group because of prolonged use of pressors with high fluid therapy 66). Therefore, a CPP >70 mm Hg is not routinely recommended in patients with TBI 9). However, no studies have been performed in patients with ICH targeting CPP ranges, and the recommendations on CPP ranges were extrapolated from a TBI guideline. A small study using comatose ICH patients showed that a CPP >80 mm Hg may be beneficial in terms of maintaining better brain tissue oxygenation, which needs to be confirmed in large numbers of patients 41). Therefore, it is still unclear which CPP goal should be suggested in patients with ICH. A variety of methods have been tried in lowering ICPs, including hyperventilation, osmotic agents (mannitol and hypertonic saline) and therapeutic hypothermia. Based on a study comparing the effectiveness of osmotic agents on ICP management using equi-osmolar doses, mannitol is considered to be equivalent to hypertonic saline. However, recent meta-analysis suggests that hypertonic saline has a tendency for better ICP control 31). In refractory cases, therapeutic hypothermia (targeting body temperature down to 33 to 35℃) may be tried to manage an ICP crisis. The effect of hypothermia on ICP lowering is considered to be comparable to osmotic therapy 67). Although some reports suggest that therapeutic hypothermia may decrease perihematomal edema, systemic infection including pneumonia is one of the common adverse events. Therefore, use of therapeutic hypothermia in ICH patients should be individualized 42).
New recommendations
1. It is recommended that patients with acute ICH are treated in the intensive care unit for the monitoring of the ICP and BP and possible need for mechanical ventilation or intubation (LOE III, GOR B) (revised recommendation).
2. Patients with a GCS score of ≤8, those with clinical evidence of transtentorial herniation, or those with significant IVH or hydrocephalus should be considered for ICP monitoring and treatment (LOE III, GOR B) (new recommendation).
3. Ventricular drainage as a treatment for hydrocephalus is reasonable in patients with a decreased level of consciousness (LOE III, GOR B) (new recommendation).
4. To control an elevated ICP, a gradual treatment should be considered. The patient's head should be elevated by approximately 30°. Limited use of analgesics and/or sedatives may be considered in patients with pain and/or unstable conditions (LOE IV, GOR C).
5. For more aggressive ICP control, the use of mannitol and hypertonic saline solution, neuromuscular blockade, hyperventilation, or therapeutic hypothermia may be tried (LOE IV, GOR C) (Revised Recommendation)
Management of seizure
Seizure is one of the common neurologic complications in patients with ICH 77). It is more common in patients with ICH compared to those with ischemic stroke, and occurs at or within 48 hours after onset. Seizure may be associated with the aggravation of the midline shift and neurologic deterioration; however, its causal relationship has not been clearly demonstrated.
1. Post-cerebral hemorrhage seizures should warrant the use of proper anticonvulsants (LOE Ib, GOR A).
2. A short-term use of anticonvulsants immediately following a lobar hemorrhage may decrease the risk of early seizures and is recommended (LOE IIa, GOR B).
3. Anticonvulsants used in cerebral hemorrhage should be gradually discontinued if no recurrence is observed. In the case of recurrence, chronic therapy may be considered (LOE IV, GOR C).
Recommendations from foreign guidelines
1. AHA/ASA52)
1) Clinical seizures should be treated with antiepileptic drugs (Class I; LOE A) (revised from the previous guideline).
2) Continuous electroencephalogram (EEG) monitoring is probably indicated in ICH patients with depressed mental status out of proportion to the degree of brain injury (Class IIa; LOE B).
3) Patients with a change in mental status who are found to have electrographic seizures on the EEG should be treated with antiepileptic drugs (Class I; LOE C).
4) Prophylactic anticonvulsant medication should not be used (Class III; LOE B) (new recommendation).
New evidences
The incidence of seizure after ICH is highly variable depending on the study population or the definition of seizure. However, clinical seizure is reported to be as high as 5% to 19% in patients with ICH within one week after onset 1,16,19,63). Moreover, electrographic seizure is four times more common than clinical seizure 16,75). In addition, 39% of patients who had electrographic seizures actually have a non-convulsive status epilepticus. The risk factors for the development of seizures after ICH are as follows : lobar in location 1,19), involvement of the cortex 19), and hematoma expansion 16). Although seizure is believed to be associated with rapid aggravation of mass effect and neurologic deterioration in patients with ICH, the causal relationship is questionable 75). Even with the development of seizures, its direct effect on poor neurologic outcome or mortality is not clear 16,19). Recently, a secondary analysis was performed using a placebo group from the Cerebral Hematoma and NXY Treatment (CHA-NT) trial, which showed that the odds for poor neurologic outcome at 90 days (mRS 5 or 6) was 6.8 in patients with prophylactic treatment using antiepileptic drugs (95% CI 2.2-21.3), sug-gesting the harmful effect of the prophylactic use of antiepileptic drugs 46). However, the majority (78%) of patients was treated with phenytoin; hence; it is not clear whether a new generation antiepileptic drugs have similar safety concerns. In a recent randomized trial, valproic acid (VPA) prophylaxis (n=36) did not prevent the occurrence of seizures compared to the placebo (n=36) in patients with spontaneous ICH 22). However, the VPA treated group did not show a poor neurologic outcome. Therefore, it is still not clear whether the use of antiepileptic drug, except for phenytoin, is associated with a poor neurologic outcome. In summary, the development of clinical seizure after ICH is probably not a cause of neurologic deterioration in patients with ICH. Moreover, the prophylactic use of antiepileptic drugs in ICH may be associated with a poor outcome, although it is still unclear whether other new generation drugs have similar safety concerns. Therefore, the prophylactic use of antiepileptic drugs without considering individual seizure risk is discouraged. However, if clinical seizure develops, it is reasonable to treat convulsive seizures with the appropriate antiepileptic drugs.
New recommendations
1. Clinical seizures should be treated with the appropriate antiepileptic drugs (LOE Ib, GOR A) (revised recommendation).
2. If the patient's mental status is depressed out of proportion to the degree of brain injury, EEG monitoring (continuous EEG, more preferably) is needed to exclude the possibility of electrographic seizures. Patients who are found to have electrographic seizures on the EEG should be treated with antiepileptic drugs (LOE III, GOR B) (new recommendation).
3. The prophylactic use of antiepileptic drugs should be individualized based on the seizure risk, and potential harmful effects of antiepileptic drugs. The use of antiepileptic drugs in all patients without considering seizure risk is not recommended (LOE IIa, GOR B) (new recommendation).
SURGICAL MANAGEMENT OF PRIMARY ICH
1. If cerebral herniation is suspected or if loss of consciousness is rapid, an early craniotomy may be considered (LOE : IV, GOR : C).
2. A craniotomy should be considered for a lobar hemorrhage located within 1 cm from the surface with a consciousness level of GCS 9-12 (LOE : IIb, GOR : B).
3. A craniotomy is recommended for a cerebellar hemorrhage of 3 cm in diameter or for symptoms suggestive of brainstem compression or hydrocephalus (LOE : IIb, GOR : B).
4. For hemorrhages located deep inside the brain, non-craniotomy surgery may be considered (LOE : IV, GOR : C).
5. For intraventricular hemorrhages, thrombolysis via ventricular puncture may be considered (LOE : IV, GOR : C).
Recommendations from foreign guidelines
1. AHA/ASA52)
1) Recommendation for the management of IVH
i. Although intraventricular administration of recombinant tissue-type plasminogen activator (tPA) in IVH appears to have a fairly low complication rate, the efficacy and safety of this treatment is uncertain and considered investigational (Class IIb; LOE B).
2) Recommendations for clot removal
i. For most patients with ICH, the usefulness of surgery is uncertain (Class IIb; LOE C). Specific exceptions to this recommendation follow.
ii. Patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression and/or hydrocephalus from ventricular obstruction should undergo surgical removal of the hemorrhage as soon as possible (Class I; LOE B). Initial treatment of these patients with ventricular drainage alone rather than surgical evacuation is not recommended (Class III; LOE C).
iii. For patients presenting with lobar clots >30 mL and within 1 cm of the surface, evacuation of the supratentorial ICH by a standard craniotomy might be considered (Class IIb; LOE B).
iv. The effectiveness of minimally invasive clot evacuation utilizing either stereotactic or endoscopic aspiration with or without thrombolytic usage is uncertain and considered investigational (Class IIb; LOE B).
v. Although theoretically attractive, no clear evidence at present indicates that ultra-early removal of the supratentorial ICH improves functional outcome or mortality rate. A very early craniotomy may be harmful due to increased risk of recurrent bleeding (Class III; LOE B).
1) Surgery is not indicated for patients with a small amount of hematoma less than 10 ml or with mild neurologic deficits (Grade D). There is no evidence for clot removal in patients with a level of consciousness of 300 on the Japan Coma Scale III (Grade C2).
2) Putaminal hemorrhage : Surgery should be considered for putaminal hemorrhage with moderate neurologic deficits or a high grade of compression due to hematoma over 30 mL (Grade C1). Especially, stereotactic clot removal is recommended in a case accompanied by a level of consciousness of 20-30 on the Japan Coma Scale II (Grade B).
3) Thalamic hemorrhage : There is no evidence for clot removal as an acute management (Grade C2). For severe ventricular enlargement as a result of ventricular rupture, ventricular drainage may be considered (Grade C1).
4) Subcortical hemorrhage : Surgery may be considered for a hematoma located 1 cm or less in depth from the cortical surface (Grade C1). A craniotomy is recommend as a surgical method (Grade C1).
5) Cerebellar hemorrhage : Surgery is indicated when there is neurologic deterioration or hydrocephalus due to compression of the brain stem in the case of a cerebellar hemorrhage 3 cm or longer in maximal diameter (Grade C1).
6) Brain stem hemorrhage : There is no evidence for clot removal in the acute stage of brain stem hemorrhage (Grade C2). Ventricular drainage may be considered when ventricular hemorrhage, as a result of brain stem hemorrhage, is the main lesion with enlarged ventricles (Grade C1).
7) IVH in adults : Evaluation of the cause of IVH is desirable when a vascular abnormality is suspected (Grade C1). Ventricular drainage should be considered when acute hydrocephalus is suspected (Grade C1).
3. European Stroke Initiative (EUSI, Europe)68)
1) Consider a craniotomy if there is deterioration in consciousness (from a GCS level of between 12 and 9 to 8 or lower), if the ICH is superficial (the clot is subcortical ≤1 cm from the surface and does not reach the deep basal ganglia) or if it is located in the cerebellum (Level C recommendation).
2) Deep-seated haematomas do not benefit from a craniotomy. Stereotaxic aspiration may be considered, especially if mass effect is present (Class IV evidence).
3) External ventricular drainage (EVD) for hydrocephalus can be ventricular or via the lumbar route if it is a communicating type of hydrocephalus. Lumbar drainage is definitely contra-indicated with all types of obstructive hydrocephalus or if the aetiology is in doubt (Class IV evidence).
4) Intraventricular thrombolysis trials may be considered if an EVD becomes necessary but not in infants (Class IV evidence).
New evidences
Removal of hematoma using a craniotomy
The result of a large multicenter trial, the STICH trial 44), which randomized 1033 patients from 107 centers over an 8-year period, beginning in 1995, was published in 2005. The inclusion criteria were 1) randomization within 72 hours and operation within 96 hours of ictus, 2) a clot over 2 cm in diameter, and 3) GCS score of 5 or more. Randomization was done to either early surgery or initial medical management if the neurosurgeon was not sure of the benefit of surgery. The primary end point of this study was the extended Glasgow Outcome Scale (GOS) at 6 months, and the secondary end point was the mortality, mRS and BI at 6 months. Five hundred six patients were randomized to surgery and 530 to medical therapy, with groups being well matched for all known variables. In an intention-to-treat analysis, surgery within 96 hours of ictus was associated with a statistically insignificant absolute benefit of 2.3% (95% CI-3.2% to 7.7%) in a 6-month prognosis-dichotomized extended GOS. The mortality [absolute benefit 1.2% (-4.9% to 7.2%)], mRS [absolute benefit 4.7% (-1.2% to 10.5%)], and BI [absolute benefit 4.1% (-1.4% to 9.5%)] showed similar statistically insignificant trends in favor of surgery. However, 26% of the medical arm ultimately crossed over to surgery because of rebleeding or neurological deterioration, and a craniotomy was performed in 85% of those crossed-over subjects. In contrast, only 75% of the patients in the primary surgical arm underwent a craniotomy, with the others being treated with less invasive surgical techniques. Ninety-three percent of the patients were available for analysis at 6 months. In addition, several subgroup analyses were performed using the age, GCS, location and amount of hematoma, distance from cortical surface, surgical method, severity of hemiplegia or aphasia, additional fibrinolytic therapy, and participating countries, which failed to show any statistically significant differences between the subgroups. However, the subgroup analysis identified that those subjects with a GCS score of 9 to 12, and those with a lobar hemorrhage with clots <1 cm from the surface may have been helped by an early craniotomy, although this did not reach statistical significance. The STICH II trial 45) started from a subgroup analysis of STICH I, which suggested benefits of early surgery for patients with a superficial lobar ICH, relative to the initial medical treatment. The STICH II included 601 patients who had a spontaneous lobar ICH with 10 to 100 mL on the CT (1 cm or less from the cortical surface of the brain), were within 48 hours of ictus, and had a best motor score with a GCS score of 5 or 6, and a best eye score of 2 or more. They were randomly assigned to early surgery or initial medical treatment. Of these patients, 291 assigned to undergo early hematoma evacuation within 12 hours of randomization, and 286 assigned to initial medical treatment were included in this analysis. The primary outcome was a prognosis-based favorable or unfavorable outcome dichotomized from the extended GOS at 6 months after randomization. Overall, 59% of the patients in the early surgery group had an unfavorable outcome at 6 months, compared with 62% of the initial medical treatment group, which was, however, statistically insignificant. Mortality, the secondary outcome, showed a reduction in the early surgery group, but this finding was also statistically insignificant. However, the subgroup analyses revealed a significant 51% relative benefit of early surgery for patients with an initial poor prognosis with a GCS score of 9-12. The STICH II concluded that early surgery did not increase mortality or morbidity at 6 months and might have a small but clinically relevant survival advantage for patients with primary superficial ICH without IVH. However, there was a large crossover in the initial medical treatment group. In STICH II, 62 (21%) of 291 patients initially assigned to medical treatment later deteriorated and underwent surgery. Although this group of crossovers had generally poor outcomes, the delayed surgery could have prevented mortality and masked the benefits of early surgery in this kind of intention-to-treat analysis. Other small randomized controlled trials (RCTs) regarding the negative results of surgical treatment for primary ICH have also been reported 30,51,80). They did not show significant improvement in the prognosis after surgical treatment for primary ICH in their RCTs for supratentorial ICH. Teernstra et al. 71) also did not provide reasonable evidence for the advantage of surgical treatment for supratentorial ICH in their meta-analysis of 9 RCTs including STICH. In contrast, a randomized study including 108 patients with supratentorial subcortical or putaminal ICH >30 mL in volume assigned to craniotomy or medical management within 8 hours of onset showed a better outcome (good recovery or moderate disability on the GCS score at 1 year) in those treated with surgery 62). However, this study showed no difference in the overall survival and had the limitation of having a small ICH volume in the patients. However, recently, individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial ICH has been published 23). This study included 2186 cases from 8 studies. Improved outcome with surgery was reported if surgery was undertaken within 8 hours of ictus, or the volume of hematoma was 20 to 50 mL, or the GCS score was between 9 and 12 in this meta-analysis. In addition, there was weaker evidence that more superficial hematomas with no IVH might also be beneficial 23). Nonrandomized clinical studies have consistently reported good outcomes for surgically treated patients with a cerebellar ICH larger than 3 cm in diameter or those with brainstem compression or hydrocephalus, whereas similar patients treated medically resulted in bad outcomes 18,36,40,53,69,73). Moreover, EVD alone instead of hematoma evacuation is not generally recommended, especially in patients with compressed cisterns 73). Surgical evacuation of thalamic and pontine ICH has been known to be limited 33,35,53). The timing of the operation remains controversial. Clinical studies have reported wide variability in the timing of the surgery, ranging from within 4 hours up to 96 hours from the onset of symptoms to the time of the operation 44,51,62,80). In a retrospective study, Kaneko et al. 34) reported on the surgical results of 100 putaminal ICHs within 7 hours of symptom onset among which sixty were within 3 hours of onset. Seven (7%) had died, whereas 15 (15%) had fully recovered, and 35 (35%) were living independently at home at 6 months after the surgery. However, subsequent randomized trials in which surgery was performed within 12 hours of onset showed mixed results 51,62,80). The main flaw of early surgery was an increased risk of rebleeding which was noted in the small trial of subjects randomized within 4 hours of onset 50). Trials that randomized patients into surgery within 24 hours 70), 48 hours 6,30), 72 hours 72,76), and 96 hours 44) have also shown no clear benefit for surgery compared with the initial medical management.
Minimally invasive surgical removal of hematoma
The rationales for minimally invasive surgery for primary ICH are 1) possibility for earlier evacuation in the clinical setting, 2) less operation time, 3) reduced trauma to the surrounding brain tissue, especially for deep seated ICH, and 4) performance under local anesthesia. In contrast, the disadvantages are 1) potential rebleeding due to the use of fibrinolytics, and 2) increased risk of infections related to prolonged indwelling catheters. The techniques for minimally invasive clot removal include stereotactic aspiration with or without thrombolytic or fibrinolytic clot lysis and endoscopic aspiration.
In their randomized study of 490 patients with putaminal ICH and moderate degree of neurological status, Hattori et al. 26) reported that stereotactic evacuation of the hematoma resulted in a lower mortality rate and better recovery to functional independence in patients with neurological Grade 3 (eyes are closed but open to strong stimuli). More recently, Wang et al. 76) reported on the results of a multicenter, randomized control clinical trial comprised 465 cases of hemorrhage in the basal ganglion from 42 hospitals in China. Three hundred and seventy-seven patients with basal ganglia ICH were randomized to minimally invasive craniopuncture therapy (n=195) or conservative control treatment (n=182). They evaluated neurological impairment on the 14th day after treatment, activities of daily living at the end of the 3rd month and the case fatality within 3 months. Improvement of neurological function in the minimally invasive craniopuncture group was significantly better than that in the control group on the 14th day. At 3 months, there was a significant difference between the two groups in the score for activities of daily living. The proportion of dependent survival patients (mRs >2) in the craniopuncture group (40.9%) was significantly lower than that in the conservative group (63.0%) at the end of the 3rd month. There was no significant difference in the cumulative fatality rates within 3 months between the two groups (6.7% in the craniopuncture group and 8.8% in the conservative group). Along with these randomized studies, a number of nonrandomized studies using the stereotactic aspiration reported aspiration rates ranging from 30% to 90% of the initial hematoma 6,12,32,37) and rebleeding rates comparable to those seen with conventional craniotomy ranging from 0% to 10% 6,12,32,37,59,60). For the Stereotactic Treatment of ICH by means of a Plasminogen Activator (SICHPA), the multicenter randomized controlled trial (n=71 patients) tried the utility of stereotactic infusion of 5000 IU of urokinase every 6 hours for a maximum of 48 hours within 72 hours of ictus 72). The inclusion criteria were 45 years or older, the amount of clot ≥10 mL, a Glasgow Eye Motor score between 2 and 10, at least one pupil reactive to light, and a normal coagulative status (corrected if necessary). Primary end points were death and the degree of functional handicap (measured with mRS) at 6 months. Overall, the mortality at 6 months was 57% ; this included 56% in the surgical group and 59% in the nonsurgical group. A significant reduction in ICH volume was achieved by the surgery (10% to 20%). Logistic regression analysis indicated the possibility of efficacy for surgical treatment, but this was not statistically significant (odds ratio, 0.23). The odds ratio of mortality combined with the mRS 5 at 180 days was also not statistically significant (odds ratio, 0.52). Since urokinase became unavailable in the United States because of reports of potential viral contamination during pharmacological preparation in 1999, tPA has been used in the treatment of ICH 10,71). Vespa et al. 74) published the phase-2 safety trial for frameless stereotactic aspiration and thrombolysis for 28 patients with ICH in the deep basal ganglia and internal capsule with a volume >5 mL. After a confirmatory CT scan to localize the catheter, 1 mg of recombinant tPA was injected into the center of the hematoma, permitted to bathe the clot for 30 minutes, and then drained into a closed circuit collection system. tPA was infused every 8 hours for 48 hours. They concluded that frameless stereotactic aspiration and thrombolysis of deep spontaneous ICH was a safe therapy that was associated with a reduction in the ICH volume, the early improvement in the National Institute of Health Stroke Scale (NIHSS) and potentially could be used to improve the outcome. Given these results, Minimally Invasive Stereotactic Surgery with rtPA for ICH Evacuation (MISTIE) study, a multicenter, randomized, controlled and stratified study comparing the administration of tPA into the clot cavity versus conventional medical treatment has recently been launched, and preliminary positive results have been published, reporting that the average clot reduction in the acute treatment phase for the surgery arm was 46% (versus the medical arm 4%) and that recorded adverse events were within safety limits, including a 30-day mortality of 8%, symptomatic rebleeding of 8%, and bacterial ventriculitis of 0% 49). The MISTIE phase II trial was designed and was done from 2005 to 2012 to find the safety and efficacy of using minimally invasive surgery with recombinant tPA. In the presentation at the International Stroke Conference 2013 reporting the results of MISTIE II, the authors reported that minimally invasive surgery with recombinant tPA showed 14% improvement in the group with a mRS 0-2, 38 fewer days for the hospital stay, savings of $44000 USD per patient, and 14% less subjects in long-term care for 365 days compared to the medical management group (unpublished yet). The MISTIE II investigators also reported that hematoma evacuation was associated with a significant reduction in perihematomal edema, one of the major causes for secondary neurologic exacerbation after ICH, and that perihematomal edema does not seem to be exacerbated by recombinant tPA 54). A controlled randomized study of endoscopic evacuation versus medical treatment was performed in 100 patients with spontaneous supratentorial intracerebral (subcortical, putaminal, and thalamic) hematomas 6). The inclusion criteria for this study were 1) patients' age between 30 and 80 years, 2) a hematoma volume of more than 10 mL, 3) the presence of neurological or consciousness impairment, 4) the appropriateness of surgery from a medical and anesthesiological point of view, and 5) the initiation of treatment within 48 hours after hemorrhage. Endoscopic hematoma removal could lower mortality in the patients with a hematoma volume ≥ 50 mL, and improve functional recovery in surgical patients with hematomas smaller than 50 mL. These effects from surgery in this study were limited to patients aged ≤60 years in a preoperatively alert or somnolent state and with a lobar hematoma. A larger volume study will clarify the benefit of endoscopic aspiration of the hematoma in patients with supratentorial ICH. Another prospective randomized study for spontaneous basal ganglia hemorrhage was performed to evaluate the safety, neurological outcomes, and cost-effectiveness of three surgical procedures : endoscopic aspiration versus stereotactic aspiration versus craniotomy 15). Ninety non-comatose patients with basal ganglia ICH were randomized into three groups. In this study, both endoscopic aspiration and stereotactic aspiration showed lower complication rates and lower mortality at 3 months and better neurologic outcomes at 6 months after surgery. However, the delayed time for stereotactic aspiration was usually longer than that of endoscopic surgery. Endoscopic surgery was more cost-effective than craniotomy using functional independence manner and BI. A meta-analysis of high-quality RCTs regarding minimally invasive surgery (stereotactic aspiration or endoscopic surgery) for spontaneous supratentorial ICH has been recently published 78). This study with 1955 patients from 12 high-quality randomized controlled trials concluded that patients with supratentorial ICH might benefit more from minimally invasive surgery than from other treatment options. The most beneficial subgroups were both sexes, 30 to 80 years of age with a superficial hematoma, a GCS score of ≥9, a hematoma volume between 25 mL and 40 mL, and within 72 hours after onset of symptoms 78).
IVH
IVH coincides with ICH in 40% of patients with primary ICH 25). IVH is comprised of primary IVH, which is confined to the ventricles, and secondary IVH which is an extension of an ICH due to hypertensive ICH or vascular malformation in the basal ganglia and the thalamus 20,25). Conventional management of IVH is the use of an EVD to remove blood clots in the ventricles and to treat obstructive hydrocephalus. Theoretically, EVD via VC can be helpful to drain blood and cerebrospinal fluid from the clotted ventricles, but an EVD alone is often inadequate for an IVH because the catheter becomes occluded with blood clots, and it takes so much time for blood clots to be clearly reduced that a longer indwelling of the catheter does not prevent inflammatory reactions caused by the breakdown of blood clots in the ventricles, which often leads to communicating hydrocephalus 29). Thus, the administration of fibrinolytic agents through a VC has attracted a lot of attention recently. Several reports from animal studies and clinical series suggested that intraventricular administration of fibrinolytic agents, including urokinase, streptokinase, and recombinant tPA, may reduce morbidity and mortality by accelerating blood clearance in IVH 21,43,55,56,58,62). The Clot Lysis, Evaluating Accelerated Resolution of IVH (CLEAR-IVH) Trial, prospectively was designed to evaluate the safety and efficacy of using 1.0 mg injections of recombinant tPA through an EVD every 8 hours up to 9 doses to accelerate lysis and evacuation of the IVH, and is still ongoing 48). In the preliminary report published in 2008, the average clot reduction after the acute treatment phase was 46%, and the 30-day mortality rate was 8%, while symptomatic rebleeding occurred in 4%, and bacterial ventriculitis in 0% 48).
New recommendations
1. If there is deterioration of consciousness due to mass effect from the hematoma, an early craniotomy may be considered (LOE IV, GOR C) (revised recommendation).
2. A craniotomy should be considered for a lobar hemorrhage located within 1 cm from the surface with a consciousness level of 9-12 on the GCS (LOE IIb, GOR B).
3. For supratentorial ICH with a GCS score of 9 or over, with a hematoma volume between 25 and 40 mL, and within 72 hours after onset of symptoms, minimally invasive surgery for evacuation of the hematoma is recommended (LOE IIb, GOR B) (new recommendation).
4. A craniotomy is recommended for a cerebellar hemorrhage with a maximal diameter of 3 cm or longer or for symptoms suggestive of brainstem compression or hydrocephalus (LOE IIb, GOR B) (revised recommendation).
5. For IVH, thrombolysis via the ventricular puncture may be considered (LOE IV, GOR C).
CONCLUSION
CPGs based on scientific evidence are presented for the medical and surgical management of patients presenting with primary ICH. This CPG describes the current pertinent recommendations and suggests Korean recommendations for the medical and surgical management of a patient with primary ICH.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI 10C2020).
References
1. Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G : Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag 2008, 4 : 715-720,    2. Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al : Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013, 368 : 2355-2365,   3. Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al : Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008, 7 : 391-399,   4. Andrews BT, Chiles BW 3rd, Olsen WL, Pitts LH : The effect of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg 1988, 69 : 518-522,   5. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) investigators Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med 2010, 38 : 637-648,    6. Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G, et al : Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma : a randomized study. J Neurosurg 1989, 70 : 530-535,   7. Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD; : STICH InvestigatorsIntraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl 2006, 96 : 65-68,   8. Brain Trauma FoundationAmerican Association of Neurological SurgeonsCongress of Neurological SurgeonsJoint Section on Neurotrauma and Critical Care, AANS/CNSBratton SL, Chestnut RM, et al : Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma 2007, 24( Suppl 1):S55-S58,   9. Brain Trauma FoundationAmerican Association of Neurological SurgeonsCongress of Neurological SurgeonsJoint Section on Neurotrauma and Critical Care, AANS/CNSBratton SL, et al : Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma 2007, 24( Suppl 1):S59-S64,   10. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al : Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007, 38 : 2001-2023,   11. Broderick JP, Adams HP Jr, Barsan W, Feinberg W, Feldmann E, Grotta J, et al : Guidelines for the management of spontaneous intracerebral hemorrhage : a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1999, 30 : 905-915,   12. Broderick JP, Brott T, Zuccarello M : Management of intracerebral hemorrhage. Edited by Batjer HH : In: Cerebrovascular Disease. Philadelphia, PA : Lippincott-Raven, 1997, pp611-627
13. Brott T, Reed RL : Intensive care for acute stroke in the community hospital setting. The first 24 hours. Stroke 1989, 20 : 694-697,   14. Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, et al : The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke 2013, 44 : 620-626,   15. Cho DY, Chen CC, Chang CS, Lee WY, Tso M : Endoscopic surgery for spontaneous basal ganglia hemorrhage : comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol 2006, 65 : 547-555; discussion 555-556,   16. Claassen J, Jetté N, Chum F, Green R, Schmidt M, Choi H, et al : Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007, 69 : 1356-1365,   17. Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC 3rd : External validation of the ICH score. Neurocrit Care 2004, 1 : 53-60,   18. Da Pian R, Bazzan A, Pasqualin A : Surgical versus medical treatment of spontaneous posterior fossa haematomas: a cooperative study on 205 cases. Neurol Res 1984, 6 : 145-151,   19. De Herdt V, Dumont F, Hénon H, Derambure P, Vonck K, Leys D, et al : Early seizures in intracerebral hemorrhage : incidence, associated factors, and outcome. Neurology 2011, 77 : 1794-1800,   20. Engelhard HH, Andrews CO, Slavin KV, Charbel FT : Current management of intraventricular hemorrhage. Surg Neurol 2003, 60 : 15-21; discussion 21-22,   21. Fountas KN, Kapsalaki EZ, Parish DC, Smith B, Smisson HF, Johnston KW, et al : Intraventricular administration of rt-PA in patients with intraventricular hemorrhage. South Med J 2005, 98 : 767-773,   22. Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y : Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res 2011, 95 : 227-231,   23. Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, et al : Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke 2012, 43 : 1496-1504,    24. Guyot LL, Dowling C, Diaz FG, Michael DB : Cerebral monitoring devices : analysis of complications. Acta Neurochir Suppl 1998, 71 : 47-49,   25. Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, et al : Intraventricular hemorrhage : Anatomic relationships and clinical implications. Neurology 2008, 70 : 848-852,    26. Hattori N, Katayama Y, Maya Y, Gatherer A : Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage : a randomized study. J Neurosurg 2004, 101 : 417-420,   27. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC : The ICH score : a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32 : 891-897,   28. Hemphill JC 3rd, Farrant M, Neill TA Jr : Prospective validation of the ICH Score for 12-month functional outcome. Neurology 2009, 73 : 1088-1094,    29. Huttner HB, Köhrmann M, Berger C, Georgiadis D, Schwab S : Influence of intraventricular hemorrhage and occlusive hydrocephalus on the long-term outcome of treated patients with basal ganglia hemorrhage : a case-control study. J Neurosurg 2006, 105 : 412-417,   30. Juvela S, Heiskanen O, Poranen A, Valtonen S, Kuurne T, Kaste M, et al : The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg 1989, 70 : 755-758,   31. Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU : Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure : a meta-analysis of randomized clinical trials. Crit Care Med 2011, 39 : 554-559,   32. Kanaya H, Kuroda K : Development in neurosurgical approaches to hypertensive intracerebral hemorrhage in Japan. Edited by Kaufman HH : In: Intracerebral hematomas. New York, NY : Raven Press, 1992, pp197-210
33. Kanaya H, Saiki I, Ohuchi T : Update on surgical treatment. Edited by Mizukami M, Kanaya K, Yamori Y : In: Hypertensive Intracerebral Hemorrhage. New York, NY : Raven Press, 1983, pp147-163
34. Kaneko M, Tanaka K, Shimada T, Sato K, Uemura K : Long-term evaluation of ultra-early operation for hypertensive intracerebral hemorrhage in 100 cases. J Neurosurg 1983, 58 : 838-842,   35. Kanno T, Sano H, Shinomiya Y, Katada K, Nagata J, Hoshino M, et al : Role of surgery in hypertensive intracerebral hematoma. A comparative study of 305 nonsurgical and 154 surgical cases. J Neurosurg 1984, 61 : 1091-1099,   36. Kase C : Cerebellar hemorrhage. Edited by Kase C, Caplan L : In: Intracerebral Hemorrhage. Boston : Butterworth-Heinemann, 1994, pp425-443
37. Kaufman HH : Stereotactic aspiration with fibrinolytic and mechanical assistance. Edited by Kaufman HH : In: Intracerebral Hematoma. New York, NY : Raven Press, 1992, pp182-185
38. Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T : Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke 1997, 28 : 2370-2375,   39. Kiening KL, Härtl R, Unterberg AW, Schneider GH, Bardt T, Lanksch WR : Brain tissue pO2-monitoring in comatose patients : implications for therapy. Neurol Res 1997, 19 : 233-240,   40. Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV : Management of spontaneous cerebellar hematomas : a prospective treatment protocol. Neurosurgery 2001, 49 : 1378-1386; discussion 1386-1387,   41. Ko SB, Choi HA, Parikh G, Helbok R, Schmidt JM, Lee K, et al : Multimodality monitoring for cerebral perfusion pressure optimization in comatose patients with intracerebral hemorrhage. Stroke 2011, 42 : 3087-3092,    42. Kollmar R, Staykov D, DÖrfler A, Schellinger PD, Schwab S, Bardutzky J : Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke 2010, 41 : 1684-1689,   43. Lapointe M, Haines S : Fibrinolytic therapy for intraventricular hemorrhage in adults. Cochrane Database Syst Rev 2002, ( 3):CD003692,    44. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al : Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005, 365 : 387-397,   45. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al : Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013, 382 : 397-408,    46. Messé SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE, et al : Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care 2009, 11 : 38-44,   47. Mokri B : The Monro-Kellie hypothesis : applications in CSF volume depletion. Neurology 2001, 56 : 1746-1748,   48. Morgan T, Awad I, Keyl P, Lane K, Hanley D : Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl 2008, 105 : 217-220,   49. Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D : Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl 2008, 105 : 147-151,   50. Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC : Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology 2001, 56 : 1294-1299,   51. Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC : Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology 1998, 51 : 1359-1363,   52. Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al : Guidelines for the management of spontaneous intracerebral hemorrhage : a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010, 41 : 2108-2129,    53. Morioka J, Fujii M, Kato S, Fujisawa H, Akimura T, Suzuki M, et al : Surgery for spontaneous intracerebral hemorrhage has greater remedial value than conservative therapy. Surg Neurol 2006, 65 : 67-72; discussion 72-73,   54. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al : Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 2013, 44 : 627-634,    55. Murry KR, Rhoney DH, Coplin WM : Urokinase in the treatment of intraventricular hemorrhage. Ann Pharmacother 1998, 32 : 256-258,   56. Naff NJ, Hanley DF, Keyl PM, Tuhrim S, Kraut M, Bederson J, et al : Intraventricular thrombolysis speeds blood clot resolution : results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery 2004, 54 : 577-583; discussion 583-584,   57. Narayan RK, Kishore PR, Becker DP, Ward JD, Enas GG, Greenberg RP, et al : Intracranial pressure : to monitor or not to monitor? A review of our experience with severe head injury. J Neurosurg 1982, 56 : 650-659,   58. Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A : Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage : a systematic review of the literature. J Neurol 2000, 247 : 117-121,   59. Niizuma H, Shimizu Y, Yonemitsu T, Nakasato N, Suzuki J : Results of stereotactic aspiration in 175 cases of putaminal hemorrhage. Neurosurgery 1989, 24 : 814-819,   60. Niizuma H, Yonemitsu T, Jokura H, Nakasato N, Suzuki J, Yoshimoto T : Stereotactic aspiration of thalamic hematoma. Overall results of 75 aspirated and 70 nonaspirated cases. Stereotact Funct Neurosurg 1990, 54-55 : 438-444,   61. Nordström CH, Reinstrup P, Xu W, Gärdenfors A, Ungerstedt U : Assessment of the lower limit for cerebral perfusion pressure in severe head injuries by bedside monitoring of regional energy metabolism. Anesthesiology 2003, 98 : 809-814,   62. Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S : Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas : a prospective randomized study. Surg Neurol 2006, 66 : 492-501; discussion 501-502,   63. Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G : Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia 2002, 43 : 1175-1180,   64. Qureshi AI, Geocadin RG, Suarez JI, Ulatowski JA : Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med 2000, 28 : 1556-1564,   65. Qureshi AI, Palesch YY : Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care 2011, 15 : 559-576,    66. Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, et al : Prevention of secondary ischemic insults after severe head injury. Crit Care Med 1999, 27 : 2086-2095,   67. Schreckinger M, Marion DW : Contemporary management of traumatic intracranial hypertension : is there a role for therapeutic hypothermia? Neurocrit Care 2009, 11 : 427-436,   68. Steiner T, Kaste M, Forsting M, Mendelow D, Kwiecinski H, Szikora I, et al : Recommendations for the management of intracranial haemorrhage - part I : spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006, 22 : 294-316,   69. Sypert G, Arpin-Sypert E : Spontaneous posterior fossa hematomas. Edited by Kaufman H : In: Intracerebral Hematomas. New York, NY : Raven Press, 1992, pp187-196
70. Tan SH, Ng PY, Yeo TT, Wong SH, Ong PL, Venketasubramanian N : Hypertensive basal ganglia hemorrhage : a prospective study comparing surgical and nonsurgical management. Surg Neurol 2001, 56 : 287-292; discussion 292-293,   71. Teernstra OP, Evers SM, Kessels AH : Meta analyses in treatment of spontaneous supratentorial intracerebral haematoma. Acta Neurochir (Wien) 2006, 148 : 521-528; discussion 528,   72. Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G, et al : Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator : a multicenter randomized controlled trial (SICHPA). Stroke 2003, 34 : 968-974,   73. van Loon J, Van Calenbergh F, Goffin J, Plets C : Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta Neurochir (Wien) 1993, 122 : 187-193,   74. Vespa P, McArthur D, Miller C, O'Phelan K, Frazee J, Kidwell C, et al : Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care 2005, 2 : 274-281,   75. Vespa PM, O'Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, et al : Acute seizures after intracerebral hemorrhage : a factor in progressive midline shift and outcome. Neurology 2003, 60 : 1441-1446,   76. Wang WZ, Jiang B, Liu HM, Li D, Lu CZ, Zhao YD, et al : Minimally invasive craniopuncture therapy vs conservative treatment for spontaneous intracerebral hemorrhage results from a randomized clinical trial in China. Int J Stroke 2009, 4 : 11-16,   77. Woo KM, Yang SY, Cho KT : Seizures after spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc 2012, 52 : 312-319,    78. Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, et al : Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage : a meta-analysis of randomized controlled trials. Stroke 2012, 43 : 2923-2930,   79. Ziai WC, Torbey MT, Naff NJ, Williams MA, Bullock R, Marmarou A, et al : Frequency of sustained intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Cerebrovasc Dis 2009, 27 : 403-410,    80. Zuccarello M, Brott T, Derex L, Kothari R, Sauerbeck L, Tew J, et al : Early surgical treatment for supratentorial intracerebral hemorrhage : a randomized feasibility study. Stroke 1999, 30 : 1833-1839,  
Table 1

Table 2
Drugs that can be used for BP control in spontaneous intracerebral hemorrhage 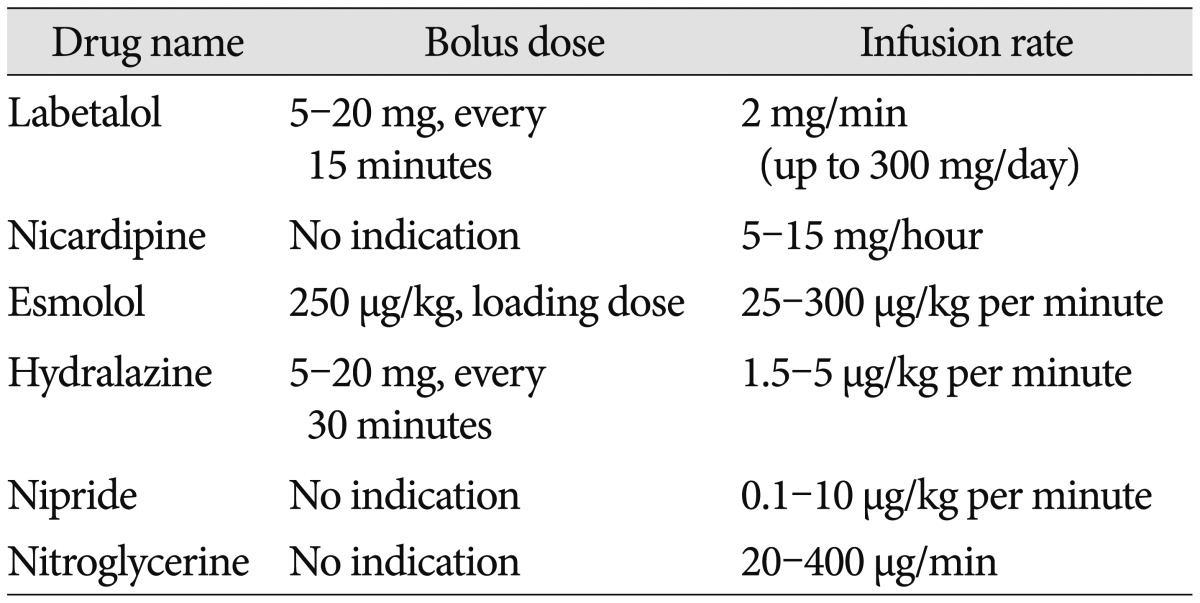
|
|

















