INTRODUCTION
Partial duplication of intracranial arteries, so called fenestration, is known to have an angiographical incidence of 0.3 to 0.9%, and is frequently associated with aneurysms22). Fenestrated arteries are often observed in the posterior circulation, but are known to be relatively unusual in the anterior circulation19). Aneurysms arising from the fenestrated A1 segment of anterior cerebral artery (ACA) are even more rare, and are considered to be unique because they may be associated with other vascular anomalies. However, the characteristics of fenestrated A1 aneurysms has not been well determined until now.
In the present report, we demonstrate a case of ruptured saccular aneurysm arising from the proximal end of a fenestrated A1 artery. By literature review, we classified fenestrated A1 aneurysm into three subtypes based on the anatomical location of the aneurysm and found the close relationship between the occurrence of fenestrated A1 aneurysm and the presence of several vascular anomalies including azygos ACA and hypoplastic A1.
CASE REPORT
Case presentation
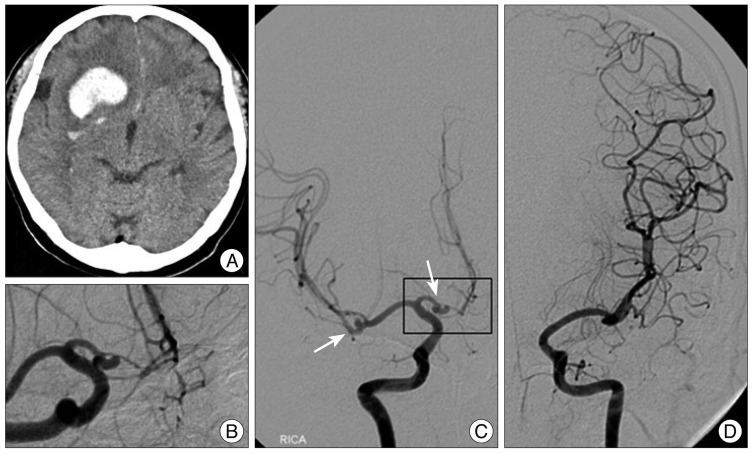
A 59-year-old woman was admitted with complaints of a sudden onset of dysarthria and left side weakness. Computed tomography revealed an intracerebral hemorrhage in the right basal ganglia and frontal lobe. Subarachnoid hemorrhage was also identified in the anterior falx and sulci of medial frontal lobes (Fig. 1A). Cerebral angiography demonstrated a saccular aneurysm which arised from the proximal end of the right fenestrated A1 segment. The A2 segment of ACA was only visible from the right internal carotid artery. There was another saccular aneurysm at the right middle cerebral artery (MCA) bifurcation which was considered as an unruptured one because of the long distance from co-existing intracerebral hematoma (Fig. 1B, C, D).
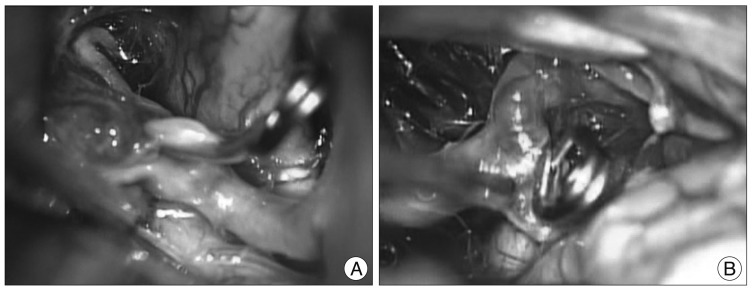
Under general anesthesia, the aneurysms were surgically clipped via right pterional craniotomy. A ruptured saccular aneurysm with broad neck was identified on the proximal end of fenestrated A1 segment intraoperatively. An aneurysm was projected anteriormedially and was occluded by Yasargil mini clip. An unruptured MCA bifurcation aneurysm was also treated by surgical clipping and the intracerebral hematoma was removed (Fig. 2).
The postoperative course was uneventful, and the patient was discharged without any neurologic deficits.
Literature review of fenestrated A1 artery aneurysms
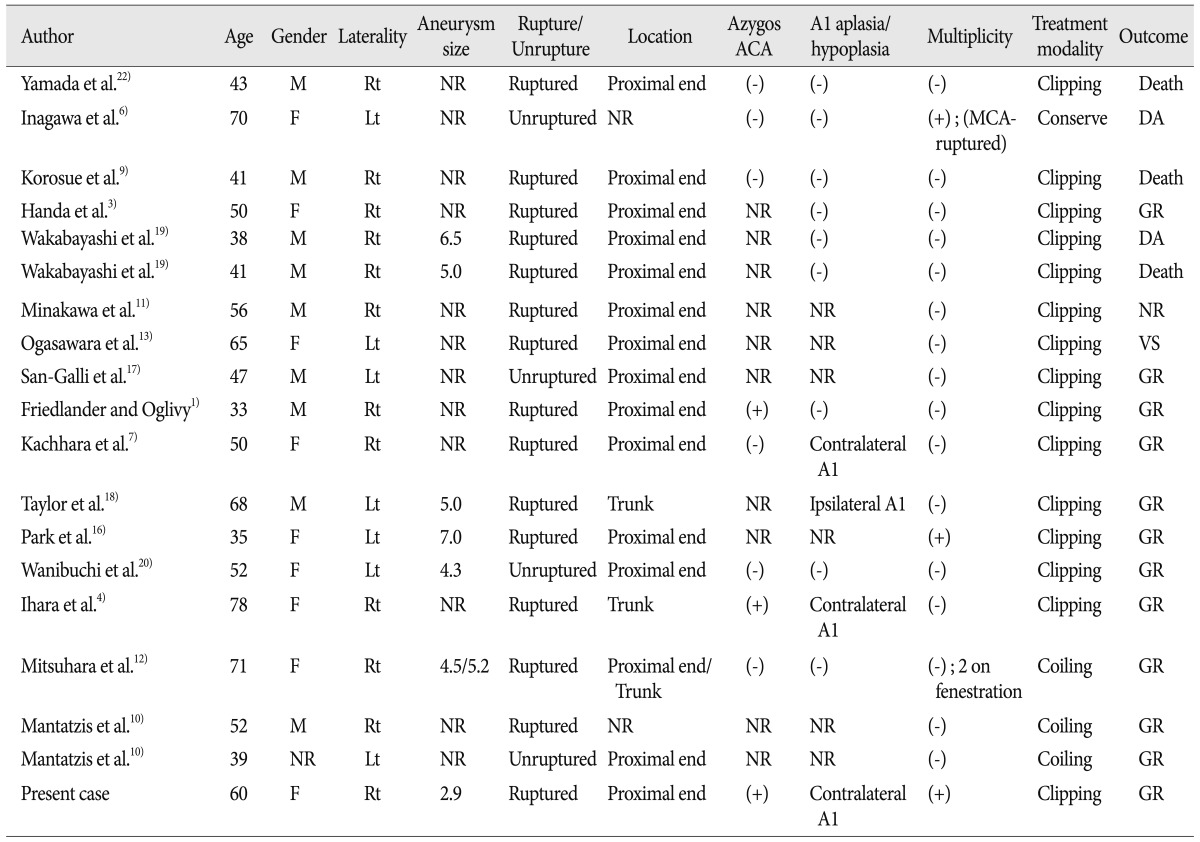
In the literature review, we found 18 cases of aneurysms arising from the fenestrated A1 segment. Characteristics of 19 cases (including present case)1-4,6,7,9-13,16-20,22) are summarized in Table 1. There were 9 males and 9 females (1 case, not recorded) with a mean age of 49.7 years (range 33-78 years). The sizes of aneurysms were recorded in 7 out of 19 cases, and the mean aneurysm size was 5.05 mm (range 2.9-7.0 mm). Twelve aneurysms were developed at the right side while seven were located at left side. All 19 aneurysms were saccular type and 15 cases (79%) were ruptured. Three among the aneurysms were treated by endovascular interventions, while the other aneurysms except for those three were treated by aneurysm clipping.
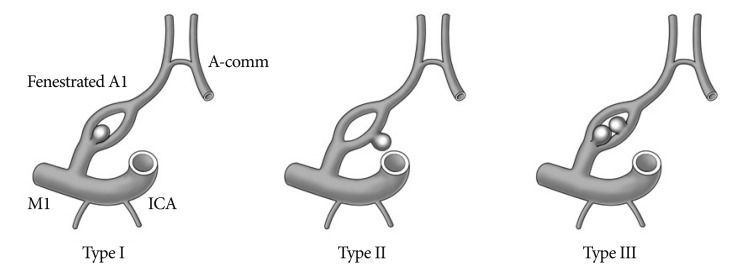
We could classify the aneurysms into three types based on the arising point of the aneurysm (Fig. 3). Of the 17 cases in which exact location of the aneurysm was described on the original article (including the present case), 14 (82%) aneurysms developed on the proximal end of the fenestrated A1 segment, which was classified as type I. Two cases (12%) were classified as type II which represent the aneurysm located on the midportion of the fenestrated A1. One case (6%) which demonstrated multiple aneurysms including one at the proximal end and another at the posterior aspect of the fenestrated artery was classified as type III. Of the nine reports in which the presence of azygos ACA has been investigated azygos ACA was noted in 3 cases (33%). Hypoplastic A1 or aplasia of A1 was demonstrated in 4 cases (31%) out of 13 cases in which the status of A1 hypoplasia or aplasia were described. In one case, the ipsilateral A1 was hypoplastic while the other three cases represented contralateral hypoplastic A1. Finally, multiple aneurysms were found in 3 cases (16%) including the present case.
DISCUSSION
There were only few articles which have proposed several theories for the development of fenestrated A1 segment. Fenestration of the A1 segment might occur due to the absence of fusion of the plexiform anastomosis which was present in the distal primitive ACA during the 18- to 43-mm stage of the embryo1,15). This failure would possibly increase blood flow in one of the A1 segments because of the contralateral A1 hypoplasia, resulting in increased hemodynamic stress on the ipsilateral A1 segment. Such increased blood flow and hemodynamic stress would prevent normal fusion of the plexiform anastomosis in the distal primitive ACA, resulting in formation of the A1 fenestration, and could also cause aneurysm formation on the fenestrated A1 segment4). However, the pathophysiologic mechanism for the development of fenestrated A1 segment is still not clearly understood.
Based on literature review, we classified fenestrated A1 aneurysm into three subtypes based on the location of aneurysms associated with A1 fenestrations, and to our knowledge, this is the first article to make a classification based on the location. In majority of the cases, the aneurysm had developed on the proximal end of the fenestration (82%), while 12% was developed on the trunk of duplicated A1. In our case, the aneurysm was located at the proximal end of the fenestrated A1, and could be determined as type I. Additionally most of fenestrated A1 aneurysms (79%) were ruptured at the time of presentation. Considering that cerebral saccular aneurysms usually arise from the arterial bifurcations where blood flow creates the greatest hemodynamic stress, the development of fenestrated A1 aneurysm also may be strongly associated with the hemodynamic force at the bifurcating point.
The overall incidence of azygos ACA is ranged from 0.22% to 1.1%2). There are two theories for azygos ACA formation. Abnormal fusion of paired A2 from the medial branch of the primitive olfactory artery at the 16-mm stage of the embryo, and persistence of the median artery of the corpus callosum at the 20- to 24-mm stage may involve in the regression or lack of development of the ACAs4,8,15). The azygos ACA is known to be closely associated with the occurrence of saccular aneurysms1,2). Considering these theories and reviewing several previous studies, we found that the incidence of azygos ACA in patients with fenestrated A1 aneurysm is much higher than the overall incidence of azygos ACA in normal population, which suggests that azygos ACA may be associated with the development of fenestrated A1 aneurysm.
The hypoplasia of the proximal ACA on one side is shown in 7-10% of general population, and is regarded as a normal variant3). However, by literature review, we found that the incidence of A1 hypoplasia or aplasia in patients with fenestrated A1 aneurysm (31%) was significantly higher than that reported in patients without aneurysms. The presence of hypoplastic A1 may lead to the increase of blood flow at the opposite side A1, in which additional hemodynamic stress may be provided. These could be one of the important predisposing factors for development of a fenestrated A1 aneurysm on the contralateral side of hypoplastic A1.
In our case, left A1 aplasia was demonstrated in cerebral angiography. In addition, there was an azygos ACA supplying blood flow to both cerebral hemispheres. Therefore, the right A1 segment was likely to receive strong hemodynamic stress, which may have affected the development of fenestrated A1 segment aneurysm and subsequent rupture.
In the literature review, multiple aneurysms were shown in 16% of patients with fenestrated A1 aneurysms, which was comparable to approximately 15-20% of incidence of multiplicity in all patients with aneurysms5,14,21). Therefore, fenestrated A1 aneurysm may not be associated with the occurrence of multiple aneurysms.
CONCLUSION
Although fenestration of the proximal anterior cerebral artery is a very rare vascular anomaly, it is likely to accompany saccular type aneurysms in the vicinity of the vascular anomaly. The fenestrated A1 aneurysm is also often co-existed with the azygos ACA or contralateral hypoplastic A1. As the aneurysms originated from fenestrated proximal ACA are prone to rupture, early treatment may benefit patients harboring those aneurysms.